
A Combined Experimental and Theoretical Investigation on the Fundamental Reaction Mechanisms of Cyanoborohydride Hypergolic Ionic Liquids with Key Oxidizers

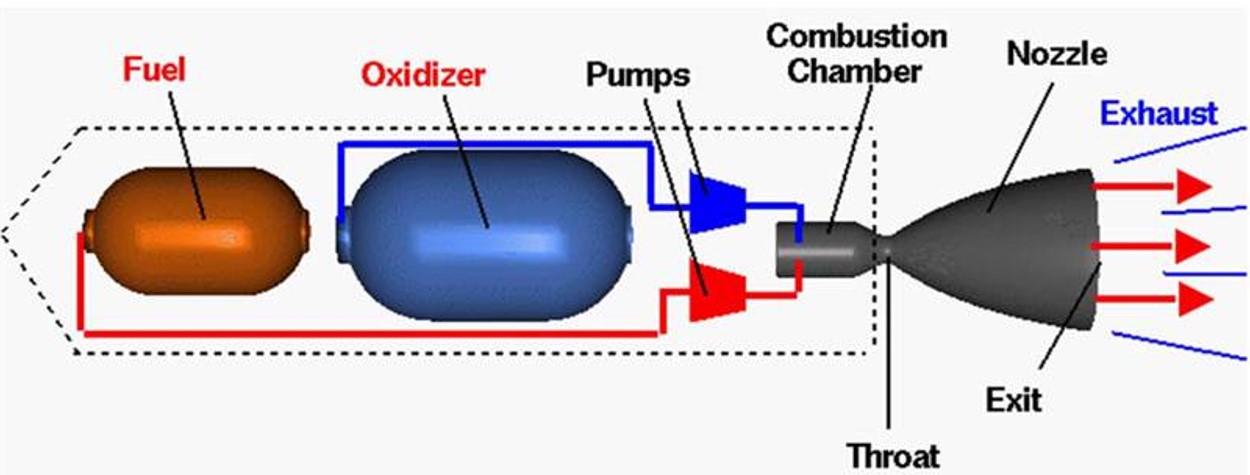
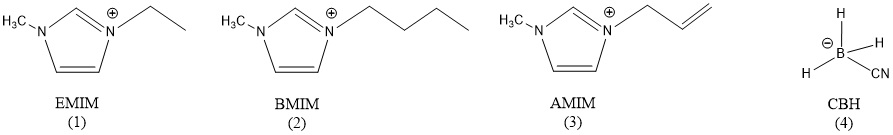
The overall goal of this project is to explore experimentally and computationally the fundamental mechanisms involved in the oxidation of prototype cyanoborohydride (CBH) hypergolic ionic liquids (HILs) with key oxidizers (HNO3, H2O2). The proposed experiment utilizes a novel droplet merging technique coupled with an ultrasonic levitation device and computations (Prof. Rui Sun, University of Hawaii) highlighting the state-of-the-art reaction dynamics simulations with machine learning and enhanced sampling techniques. These findings are essential to the Air Force in the development of next-generation hypergolic ionic liquid-based chemical propulsion systems through pioneering, fundamental research including novel diagnostics technology and computational developments. These objectives are achieved by systematically initiating the reaction and ignition of HILs by merging levitated droplets of HILs with droplets of the oxidizer within a novel ultrasonic levitation device at relevant temperatures and pressures under container-less conditions - an emerging laboratory technique, which has not been accessible in previous HIL studies. The oxidation reaction between the HILs and oxidizers will be also unraveled at an atomistic level with ab initio molecule dynamics simulation accelerated by a novel machine learning technique; the condensed phased environment will be modeled in the presence of explicated solvent with cutting-edge enhanced sampling method. The tight experiment-computation collaboration proposed in this project determines hypergolic reaction mechanisms of ionic liquids and their dependencies on the oxidizer, identifies reaction critical intermediates that could explain the differences in ignition delays and defines the functional groups, which need to be present in the structure core of the ionic liquid, to enable ignition. Overall, this research will shed unprecedented light on the interplay between the structure of the HILs and its hypergolicity and provides insights into the mechanism of the ignition delay on the molecular level.
Recent Selected Publications
1. K. Fujioka, R. I. Kaiser, R. Sun, Unsupervised Reaction Pathways Search for the Oxidation of Hypergolic Ionic Liquids: 1‑Ethyl-3-methylimidazolium Cyanoborohydride (EMIM+/CBH−) as a Case Study, J. Phys. Chem. A, 127, 913-923 (2023). (PDF) (Supplemental Information)
2. S. Biswas, I. Antonov, K. Fujioka, G. L. Rizzo, S. D. Chambreau, S. Schneider, R. Sun, R. I. Kaiser, Unraveling the initial steps of the ignition chemistry of the hypergolic ionic liquid 1-ethyl-3-methylimidazolium cyanoborohydride ([EMIM][CBH]) with nitric acid (HNO3) exploiting chirped pulse triggered droplet merging, Phys. Chem. Chem. Phys., 25, 6602-6625 (2023). (PDF) (Supplemental Information)
3. S. Biswas, K. Fujioka, I. Antonov, G. L. Rizzo, S. D. Chambreau, S. Schneider, R. Sun, R. I. Kaiser, Hypergolic ionic liquids: to be or not to be?, Chem. Sci., 15, 1480−1487 (2024). (PDF) (Supplemental Information)
4. S. Biswas, K. Fujioka, D. Paul, M. Mcanally, G. L. Rizzo, S. D. Chambreau, S. Schneider, R. Sun, R. I. Kaiser, Unraveling the Unusual Chemistry of the Hydrogen-Peroxide-Driven Hypergolic Ignition of a Cyanoborohydride Ionic Liquid as a Next-Generation Green Space Propellant, J. Phys. Chem. Lett., 16, 1831−1839 (2025). (PDF) (Supplemental Information)
5. S. Biswas, M. Mcanally, S. D. Chambreau, S. Schneider, R. Sun, R. I. Kaiser, Atmospheric Ignition Chemistry of Green Hypergolic Bipropellant 1-Ethyl-3-Methylimidazolium Cyanoborohydride – Hydrogen Peroxide in an Acoustic Levitator: Exploring a Potent Universal Propellant, Chem. Eur. J., 31, e202500593 (2025). (PDF) (Supplemental Information)

