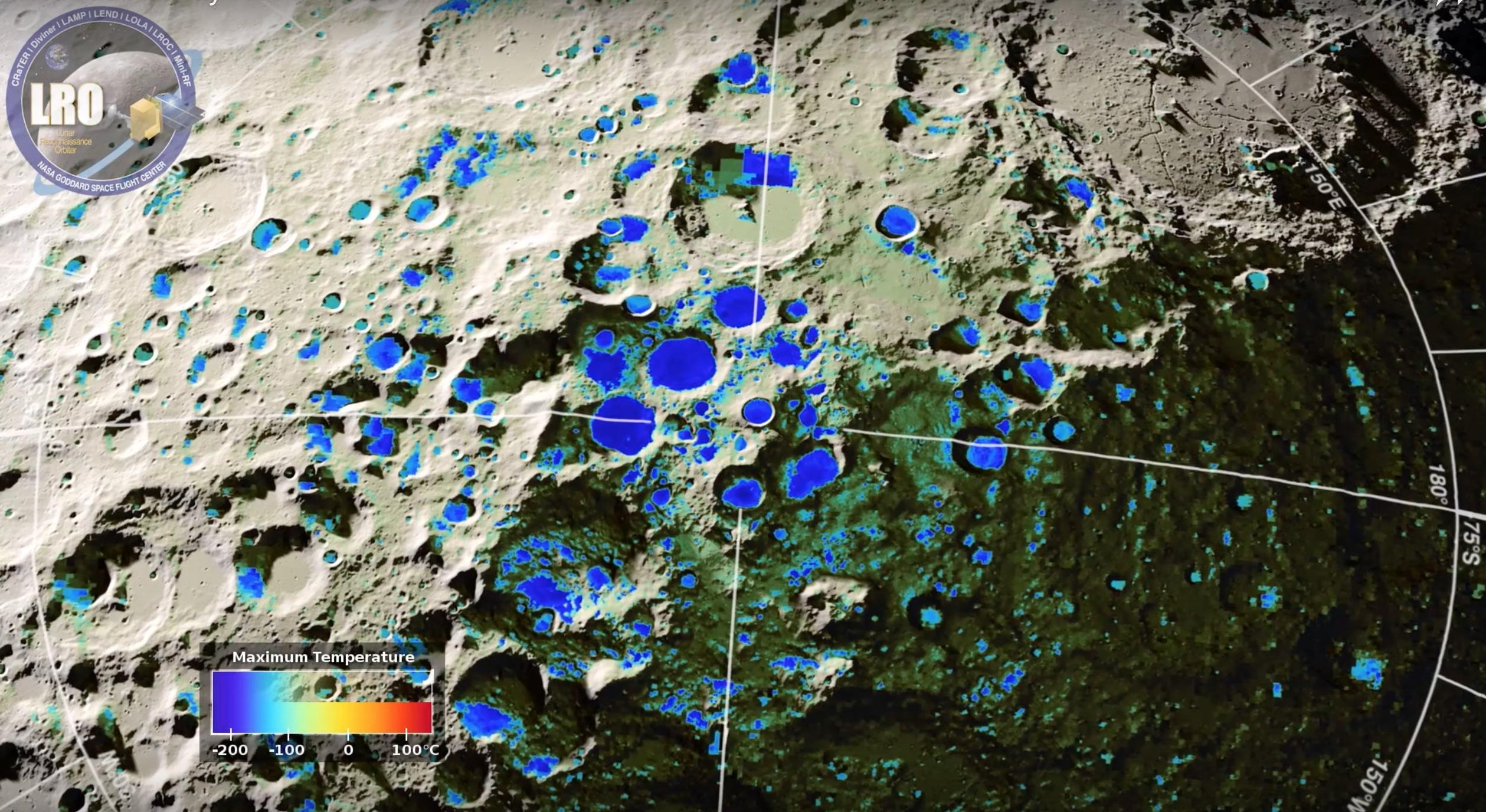
The overall goal of this project is to investigate the in situ formation of water along with its destruction mechanisms on the lunar surface. The surface of the moon, as well as all airless bodies, interacts with the solar wind to create and/or destroy volatiles such as water. This chemically active boundary surface is important to characterize because it is the part that is observed with remote sensing instruments and has and will be sampled/tested/utilized as a resource by robotic instruments and astronauts. It also may be the catalyst where much of the water ice found in permanently shadowed regions is generated. Hence, determining the chemical stability of water (H2O) and hydroxyl radicals (OH) produced via the solar wind is important for understanding the origin, evolution, and accessibility of this potential resource for human exploration. Evidence of widespread H2O and OH in the lunar surface layer has been an important discovery for Moon Mineralogy Mapper (M3) with additional supporting observations by VIMS and EPOXI. While the interpretation of OH is fairly well accepted, the presence of H2O is much less certain. Currently, no data exists on the production or desorption rates of H2O and OH under simulated lunar condition: vacuum, temperatures ranging from permanent shadowed regions (50 K) to lunar noon (400 K), irradiation of protons and photons. Consequently, we are proposing to fill the significant gap of knowledge pertaining specifically to in situ formation and destruction mechanisms of water and the hydroxyl radicals. These experiments are carried out in collaboration with Prof. Jeffrey Gillis-Davis (HIGP, UH Manoa).

Recent Selected Publications
1. C.J. Bennett, C.P. Enis, R.I. Kaiser, Experimental Studies on the Formation of D2O and D2O2 by Implantation of Energetic D+ Ions into Oxygen Ices, The Astrophysical Journal, 782, 63 (2014). (PDF)
2. C.J. Bennett, C.P. Ennis, R.I. Kaiser, Implantation of Energetic D+ Ions into Carbon Dioxide Ices and Implications to our Solar System: Formation of D2O and D2CO3, Ap. J., 974, 57 (2014). (PDF)
3. P.B. Crandall, S. Góbi, J. Gillis-Davis, R.I. Kaiser, Can perchlorates be transformed to hydrogen peroxide (H2O2) products by cosmic rays on the Martian surface?, J. Geophys. Res. Planets, 122, 1880-1892 (2017). (PDF)
4. C. Zhu, P. B. Crandall, J. J. Gillis-Davis, H. A. Ishii, J. P. Bradley, L. M. Corley, R. I. Kaiser, Untangling the Formation and Liberation of Water in the Lunar Regolith, Proceedings of the National Academy of Sciences, 116, 11165-11170 (2019). (PDF)
5. C. Zhu, S. Góbi, M. J. Abplanalp, R. Frigge, J. J. Gillis-Davis, G. Dominguez, K. Miljkovic, R. I. Kaiser Regenerative water sources on surfaces of airless bodies, Nat. Astron., 4, 45-52 (2020). (PDF)
6. P. B. Crandall, J. J. Gillis-Davis, R. I. Kaiser, Untangling the Origin of Molecular Hydrogen in the Lunar Exosphere, Astrophys. J. 887, 1 (2019). (PDF)
7. C. Zhu, S. Góbi, M. J. Abplanalp, R. Frigge, J. J. Gillis-Davis, R. I. Kaiser, Space Weathering-Induced Formation of Hydrogen Sulfide (H2S) and Hydrogen Disulfide (H2S2) in the Murchison Meteorite, J. Geophys. Res.-Planet. 124, 2772-2779 (2019). (PDF) (Supplemental Information)


