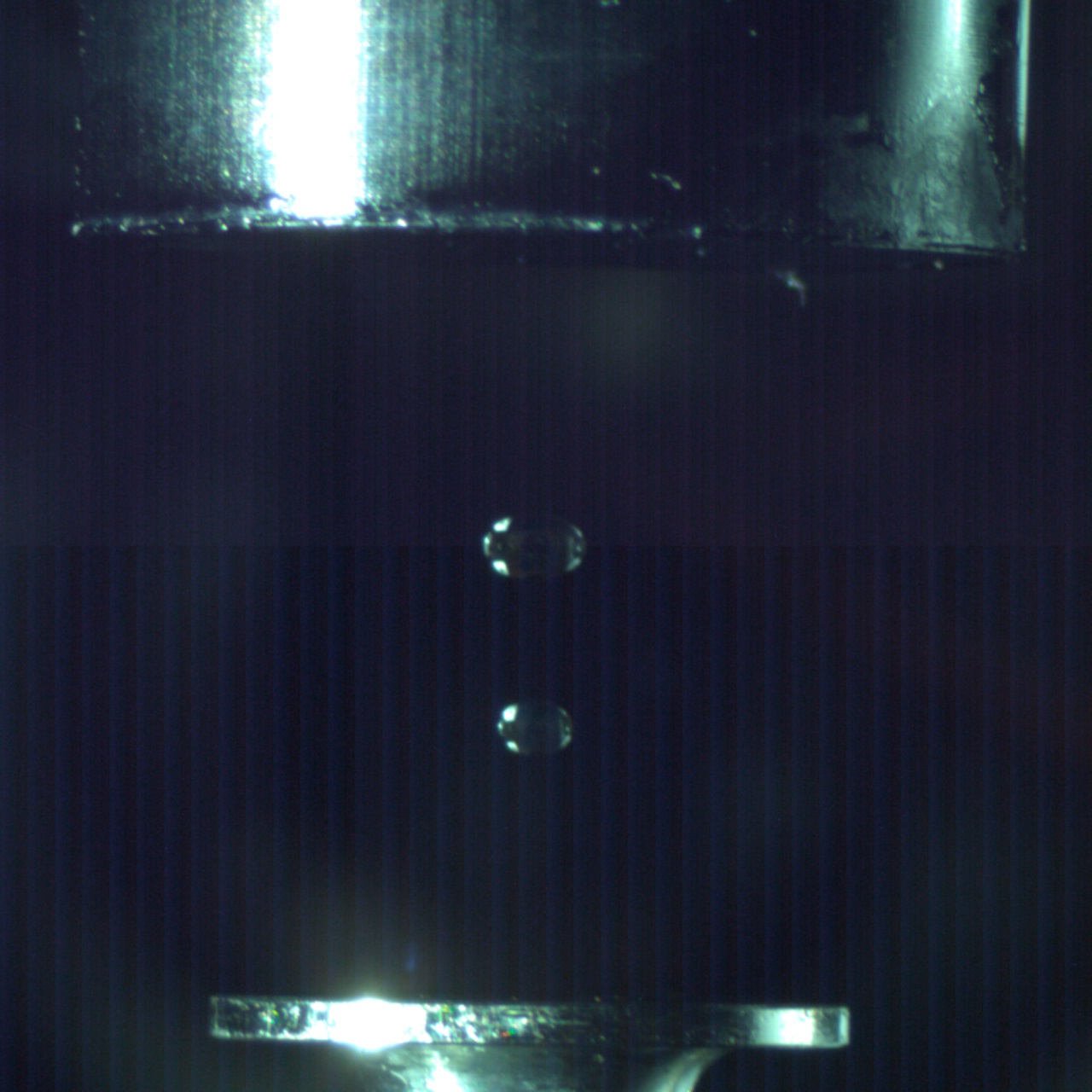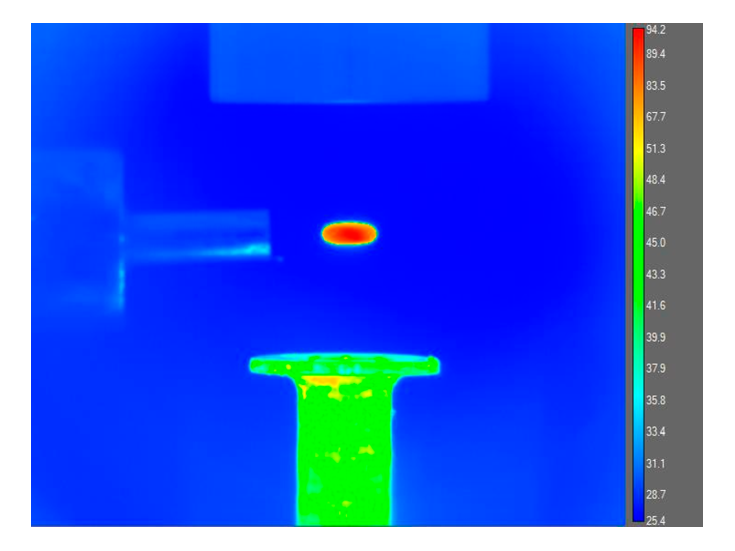

An ultrasonic levitation device coupled to a pressure-compatible process chamber helps studying hetero-geneous reactions on freely levitated soot analog particles leading to the formation of polycyclic aromatic hydrocarbon molecules and soot at temperatures from 300K to a few 1,000K and pressured from 10 torr to 2280 torr. By heating the levitated particles to combustion-relevant temperatures with the help of a carbon dioxide laser, surface reactions with aromatic (phenyl) and resonantly stabilized free radicals (propargyl, allyl) from the gas phase are triggered. Here, acoustic levitation is based on the production of a standing wave with equally spaced nodes and antinodes by multiple reflections between an ultrasonic radiator and solid reflector. When a particle is suspended in a vertically oriented sound field, the sound exerts a pressure counteracting the gravitational force. Particles with sizes between a few tens of micrometers up to millimeters can be trapped in the pressure nodes of a standing ultrasonic wave. Therefore, ultrasonic levitation can be utilized to avoid solid walls and macroscopic surfaces around a freely suspended sample as the only contact is the surrounding medium (air, inert gas). Acoustic levitation has the advantage of not requiring any defined physical properties of the samples (e.g. electric charge, refractive index). This makes acoustic levitation a versatile tool.


Utilizing cryo-cooling, this setup is also capable to investigate the photochemical processing of (ice coated) micrometer sized organic and mineral-based grain particles as analog particles of interplanetary grains, cometary dust, and planetary ring particles at temperatures as low as about 80K. This machine will be interfaced to complimentary, non-evasive spectroscopic probes (Infrared, Raman, UVVIS) thus enabling us to chemically characterize the levitated particles and their chemical modifications. The study of heterogeneous reactions on soot analog material has been conducted extensively on macroscopic surfaces utilizing standard surface science techniques such as surface scattering experiments in which, for instance, a supersonic radical beam interacts with the macroscopic surface. However, the physical and chemical processes on macroscopic surfaces do not necessarily mimic that of surfaces found on soot particles. For instance, it is known from atmospheric sciences, that the ozone depletion of photochemical smog is significantly different on micrometer sized particles compared to bulk surfaces in the laboratory. The role of diffusion and heat transfer has minimal consequences on bulk surfaces, whilst cumulative effects on micron-sized grains are significant due to their distinct surface area. Therefore, the chemical processing and growth of soot particles cannot be always studied by extrapolating data from bulk surface, and a novel experimental approach, which overcomes these limitations, as presented here is desired.