The exploration of the deuterium fractionation in the interstellar medium presents an important means to examine chemical models on the evolution of cold molecular clouds and of carbon rich circumstellar envelopes. Compared to the interstellar D/H abundances of about 10-5, deuterium enrichments up to a factor of 103 can be observed in interstellar molecules. So far, about 32 isotopomers have been detected ranging from mono-, di-, to triply deuterated molecules. These are, for instance, the singly deuterated species D-thioformaldehyde (HDCS), D-ethynyl (CCD), D-water (HDO), D-cyclopropenylidene (c-C3HD), D-tricarbonhydride (C3D), D2-ammonia (NH2D), D-methanol (CH2DOH), D-formaldehyde (HDCO), D-hydrogencyanide (DCN), D-iso hydrogencyanide (DNC), D-cyanoacetylene (DCCCN), D-cyanodiacetylene (DCCCCCN), D-methylacetylene (CH2DCCH), D-hydrogen sulfide (HDS), D-hydrogen (HD) together with ions H2D+, DCO+, and DN2+. D2-formaldehyde (D2CO), D2-methanol (CHD2OH), D2-hydrogesulfide (D2S), D2-ammonia (ND2H), and D2-HD2+ are the only doubly deuterated species observed so far. The recent detection of the triply deuterated molecules D3-ammonia (ND3) and D3-methanol (CD3OH) yielded important clues on the relative importance of gas phase versus grain – surface reactions and their role in the deuterium enrichment of interstellar molecules.


In the gas phase, it is generally accepted that the essential principle leading to a deuterium enrichment involves exothermic ion-neutral reactions under thermodynamical equilibrium which have no entrance barrier. But despite elaborate ion-neutral models, the mechanism leading to a deuterium-enrichment in complex organic, hydrogen-deficient molecules like c/l-C3H2 and C3H is far from being. For instance, the synthesis of c-C3H2 and c-C3DH is suggested to involve an elusive C3H2D+ intermediate which fragments upon dissociative recombination to c-C3H2/C3HD and C3D/C3H. However, the D/H ratio of 0.1 as found toward the molecular cloud TMC-1 could not be reproduced with this ion-neutral scheme; only D/H ratios between 0.004 and 0.02 were obtained in chemical reaction models simulating the deuterium fractionation in TMC-1. An alternative pathway leading to a deuterium enrichment at low temperatures could implicate rapid and barrierless neutral-neutral reactions. A recent theoretical study of the neutral-neutral reaction of carbon atoms in their C(3Pj) ground state reacting with D1-acetylene (DCCH) suggest that the formation of the linear isotopomer, l-C3H, plus atomic deuterium is endothermic by 5.8 kJmol-1; on the other hand, the reaction to yield l-C3D plus atomic hydrogen was found to be exothermic by 0.3 kJmol-1. Therefore, this process can support a deuterium enrichment in the l-C3D isomer at low temperatures as prevailing in molecular clouds such as TMC-1.
Despite these progresses, the deuterium enrichment in more complex organic radicals like D-1,3-butadiynyl (CCCCD) as observed by Turner has not been fully understood yet. This is pretty surprising, since the corresponding 1,3-butadiynyl radical (CCCCH) in its 2Σ+ electronic ground state has received considerable attention due to its potential significance as a precursor to polycyclic aromatic hydrocarbons (PAHs) and possibly to fullerenes in the interstellar medium. However, recent crossed molecular beams experiments of dicarbon molecules (C2) with acetylene (HCCH) suggest that the 1,3-butadiynyl radical, CCCCH (X2Σ+), can be formed in an exoergic and barrier-less reaction under single collision conditions via a dicarbon versus atomic hydrogen exchange pathway following indirect scattering dynamics through cyclic and acyclic C4H2 reaction intermediates.
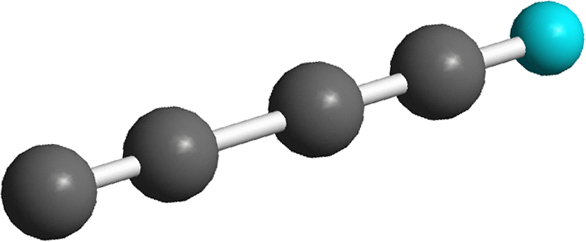
Here, the reaction of the simplest carbon cluster, dicarbon, with D1-acetylene (HCCD) was examined under single collision conditions in a crossed beams machine and computationally to investigate deuterium enrichment processes in cold molecular clouds and in circumstellar envelopes of carbon stars. Our studies suggest that this barrier-less neutral – neutral reaction can induce a deuterium enrichment in the D1-1,3-butadiynyl radial (CCCCD) over a broad range of collision energies corresponding to temperatures from 10 K to a few 1000 K. We also extracted a versatile concept suggesting that each rapid neutral-neutral reaction, which proceeds via indirect scattering dynamics through a reaction intermediate residing in a deep potential energy, can lead to a deuterium enrichment even at elevated temperatures of a few 1000 K as present, for instance, in the envelope of the dying carbon star IRC+10216. These findings should trigger an astronomical search for the CCCD molecule in the circumstellar shells of IRC+10216 and an update of chemical models simulating the deuterium enrichment in cold molecular clouds and dying carbon stars.
Recent Selected Publications
1. Y. Guo, V.V. Kislov, X. Gu, F. Zhang, A.M. Mebel, R.I. Kaiser, A Combined Experimental and Theoretical Study of the Reaction of Dicarbon (C2) with D1-Acetylene (HCCD): Possible Mechanisms for Deuterium enrichment in the interstellar D1-Butadiynyl Radical, (CCCCD(X2Σ+)), Ap. J., 653, 1577-1582 (2006). (PDF)


