For more than four decades, the structural isomers of the carbon trioxide molecule (CO3) have received considerable attention. Whether considering chemistry in the atmospheres of Venus, Earth, or Mars, or in the ices of the Martian polar caps and in comets, these isomers are essential in understanding non-thermal chemistry of the carbon dioxide molecule (CO2) and the inherent 18O isotope enrichment of stratospheric carbon dioxide.[1-4] In view of radiation-induced degradation of oxygen-bearing molecules like ozone or carbon dioxide, ionizing radiation from the solar wind can liberate oxygen atoms in their electronic ground (3P) or first electronically excited state (1D) both with a significant amount of excess kinetic energy. These atoms were suggested to react with carbon dioxide molecules either in the gas phase or in the solid state to form a carbon trioxide intermediate(s). Here red atoms indicate oxygen where gray atoms are carbon. An isotopically labeled oxygen (18O) reactant has been introduced to keep track of the isotope exchange pathways.
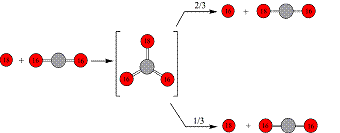

If the CO3 intermediate is not stabilized by a third body collision, it may dissociate into oxygen and carbon dioxide, possibly quenching an electronically excited oxygen atom or undergoing isotope exchange of the oxygen atoms. These pathways have been included in atmospheric models to explain the 18O isotope enrichment of stratospheric carbon dioxide on Earth and the regeneration of carbon dioxide on Mars, both in the upper atmosphere and catalyzed in solid carbon dioxide ices. However, despite the importance of the carbon trioxide isomers in atmospheric chemistry, only the C2v symmetric structure has been observed experimentally so far via low temperature spectroscopy. The D3h isomer lies only 0.4 kJ mol-1 higher in energy than the C2v structure with an isomerization barrier of 18.4 kJ mol-1. Evidence for an interconversion of both structures first came when Baulch and Breckenridge, using isotopic labeling, found that an attacking oxygen atom has an approximately statistical (2/3) chance of being incorporated into the carbon dioxide molecule. With the long lifetime of the CO3 intermediate, sufficient time could exist to access the D3h structure and equally distribute the excess energy into the internal modes of freedom giving each oxygen atom a nearly statistical probability of being ejected from the D3h isomer. However, the inclusion of the D3h isomer into the 18O isotope exchange models is only theoretical, making an investigation into the physical properties of the CO3 intermediate and the elementary mechanisms and kinetics of the reactions involved in 18O isotope enrichment difficult. Consequently, confirming the existence of the D3h isomer would be a crucial step in validating this isomerization mechanism from the C2v structure that is necessary in explaining the isotope exchange experiments. Also, if a one-step mechanism of formation of the D3h isomer was found via the reaction of an oxygen atom with a carbon dioxide molecule, it would provide an alternative, hitherto neglected pathway for quenching O(1D) atoms and 18O enrichment in the atmospheres of Earth, Mars, and Venus. Here, we report on the first spectroscopic detection of the acyclic (D3h) isomer of carbon trioxide (12C16O3) via its v1 and v2 vibrational modes centered around 1165 cm-1 under matrix isolation conditions; the identification of the 12C18O3, 13C16O3, 13C18O3, 16O12C18O2, and 18O12C16O2 isotopomers of the acyclic isomer confirms the assignments.
Recent Selected Publications
1. C.J. Bennett, C. Jamieson, A.M. Mebel, R.I. Kaiser, Untangling the formation of the cyclic carbon trioxide isomer in low temperature carbon dioxide ices, Phys. Chem. Chem. Phys. 6, 735-746 (2004). (PDF)
2. C.S. Jamieson, A.M. Mebel, R.I. Kaiser, Identification of the D3h isomer of carbon trioxide (CO3) and its implications for atmospheric chemistry, Chem. Phys. Chem., 7, 2508-2513 (2006). (PDF)
3. C.S. Jamieson, A.M. Mebel, R.I. Kaiser Investigating the Formation of Intermediates in the Reactions of Carbon dioxide (CO2) with Suprathermal Oxygen and Nitrogen Atoms, in: Astrochemistry - From Laboratory Studies to Observations. American Institute of Physics, 855, 100-106 (2006).
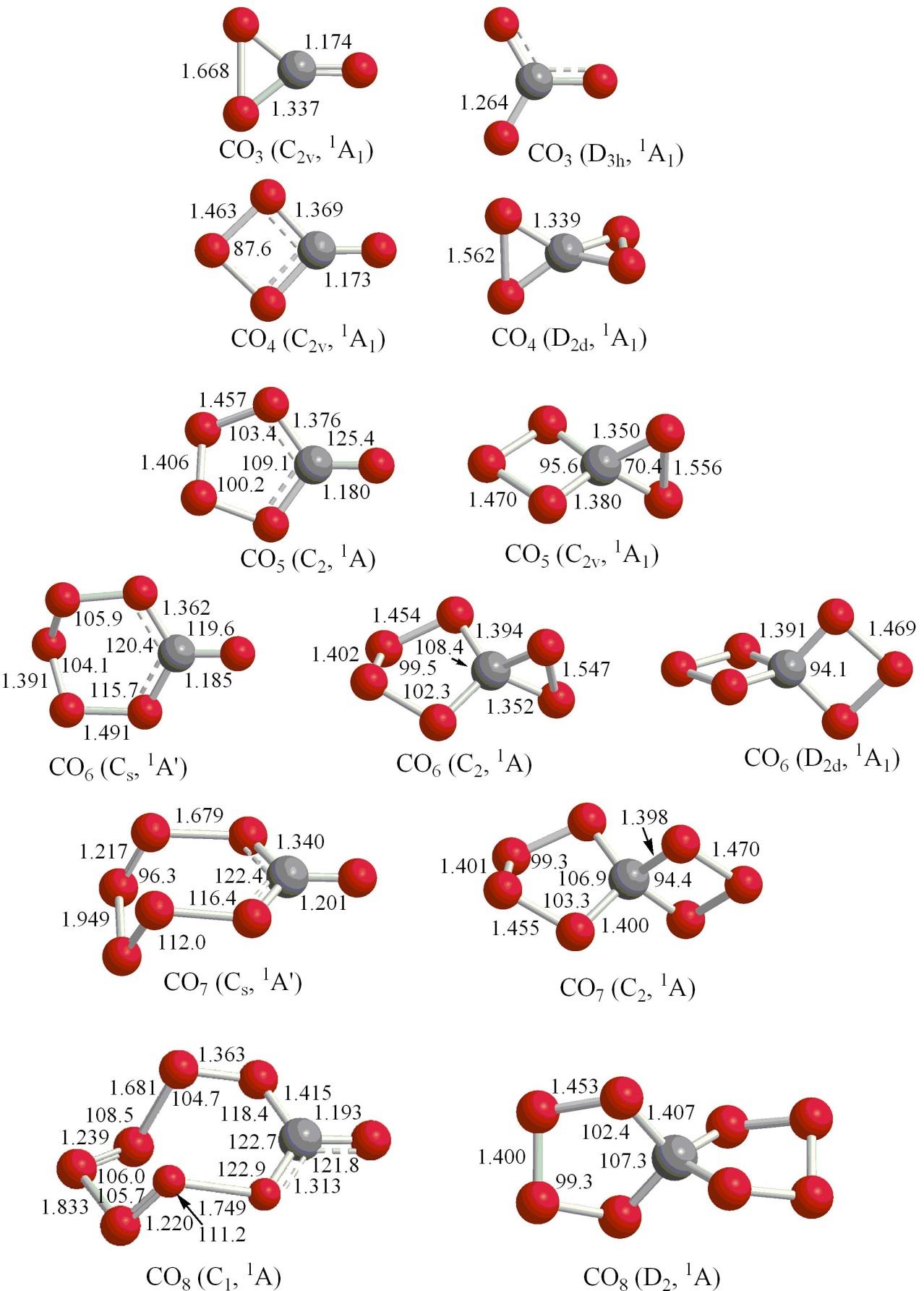
4. C.S. Jamieson, A.M. Mebel, R.I. Kaiser, Novel Detection of the C2v Isomer of Carbon Tetraoxide (CO4), CPL 440, 105-109 (2007). (PDF)
5. C.S. Jamieson, A.M. Mebel, R.I. Kaiser, First detection of the C2 symmetric Isomer of Carbon Pentaoxide (CO5) at 10 K, Chemical Physics Lett. 443, 49-54 (2007). (PDF)
6. C.S. Jamieson, A.M. Mebel, R.I. Kaiser, First detection of the Cs Symmetric Isomer of Carbon Hexaoxide (CO6) at 10 K, Chem. Phys. Lett 450, 312-317 (2008). (PDF)
7. R.I. Kaiser, A.M. Mebel, On the Formation of Higher Carbon Oxides in Extreme Environments, Invited Frontiers Article. Chem. Phys. Lett. 465, 1-9 (2008). (PDF)
8. C.J. Bennett, C.S. Jamieson, R.I. Kaiser, Mechanistical Studies on the Formation and Destruction of Carbon Monoxide (CO), Carbon Dioxide (CO2), and Carbon Trioxide (CO3) in Interstellar Ice Analog Samples, PCCP 12, 4032-4050 (2010). (PDF)
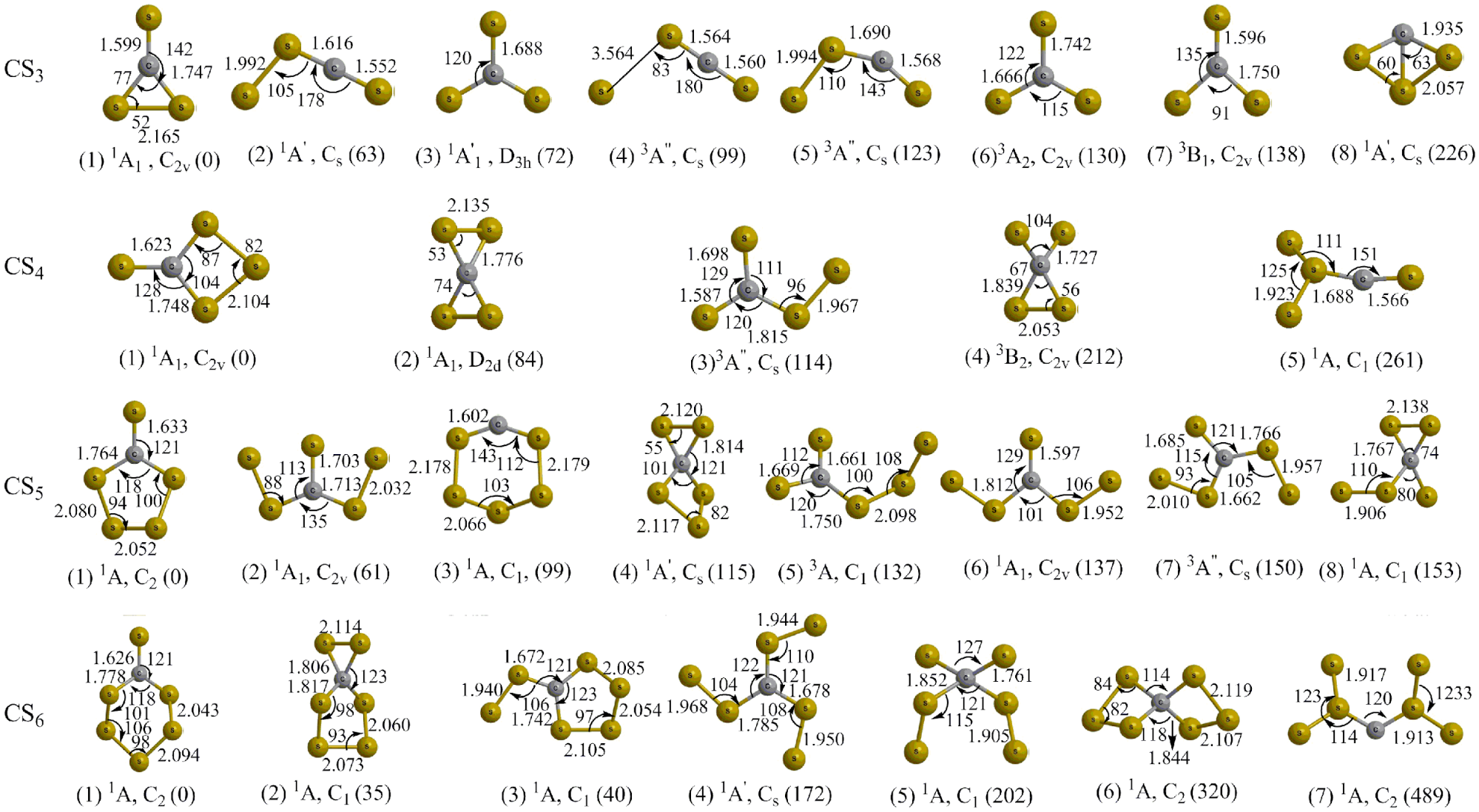
9. S. Maity, Y.S. Kim, R.I. Kaiser, H.C.C. Chang, On the Detection of Higher Order Carbon Sulfides (CSx ; x = 4-6) in Low Temperature Carbon Disulfide Ices, CPL 577, 42-47 (2013). (PDF)


