Reactive scattering experiments are conducted under single collision conditions to elucidate the intimate details of a chemical reaction such as the reaction mechanism, entrance and exit barriers, and reaction intermediates involved. This would not be possible in bulk experiments such as in test tubes where wall effects and three body collisions influence the outcome of a chemical reaction. Our experiments are carried out in a crossed molecular beams apparatus, where two supersonic, pulsed beams of reactants collide under well defined conditions. The resulting products are analyzed in a triply differentially pumped rotatable detector after ionization of the neutral molecules utilizing (tunable) electron impact ionization. Different supersonic beam sources are available ranging from photolytic, pyrolytic, and ablation sources. To gain additional information on the reaction products, partially isotopically labeled species can be utilized to pin down, for instance, the position of the hydrogen versus deuterium atom loss. These experiments are conveniently conducted due to pulsed molecular beams which can limit the consumption of expensive, thermally unstable, and also toxic chemicals.
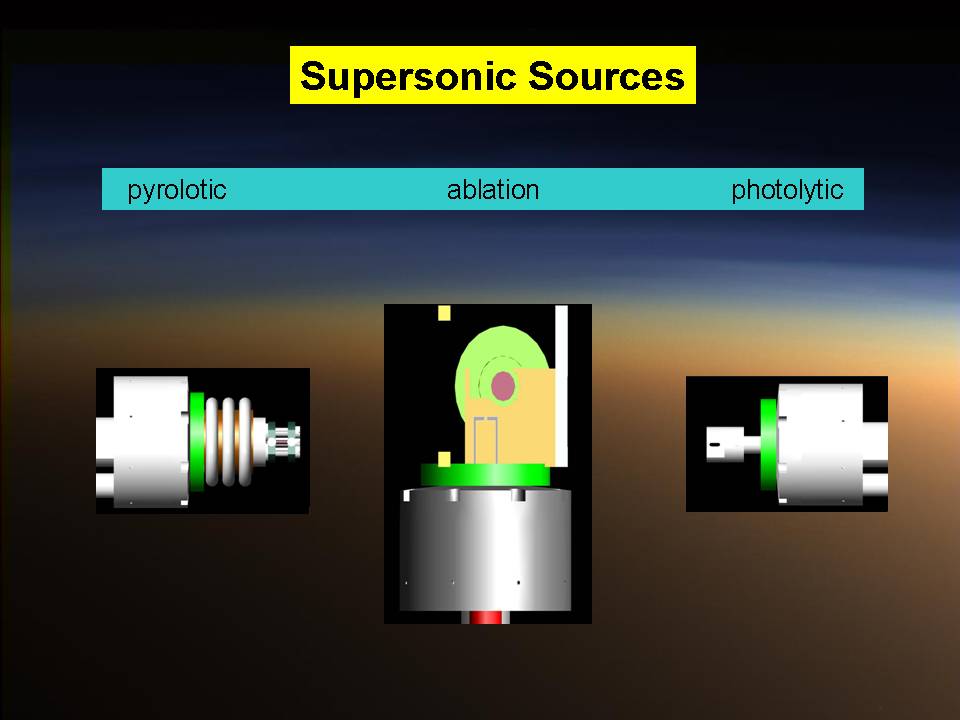
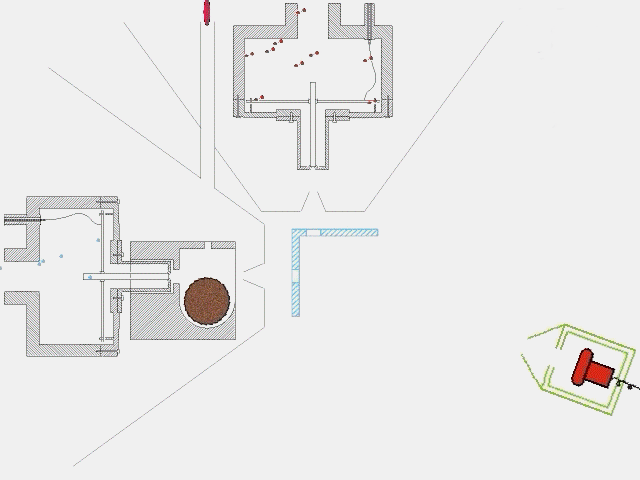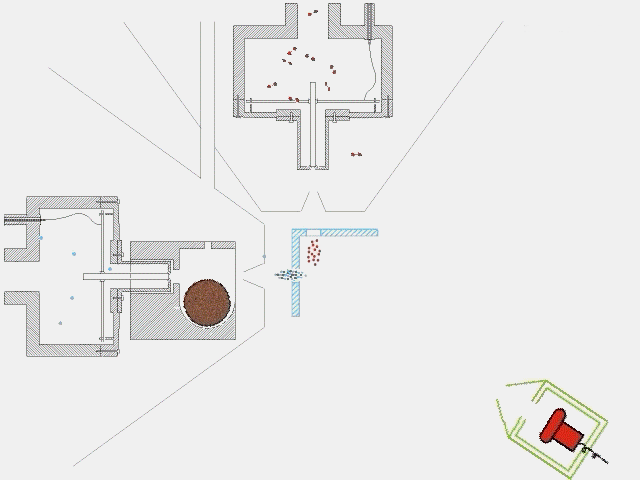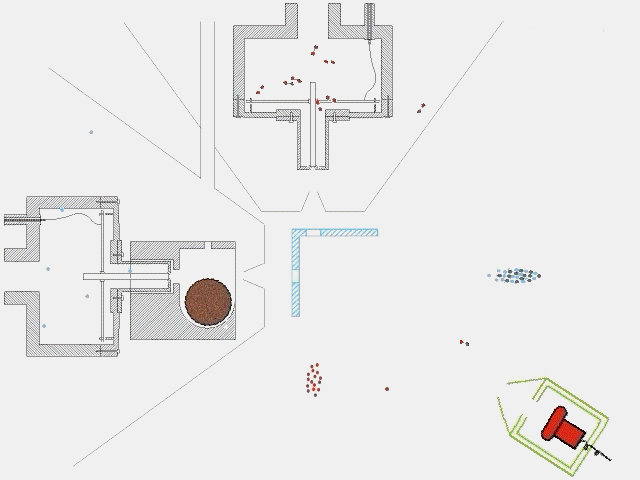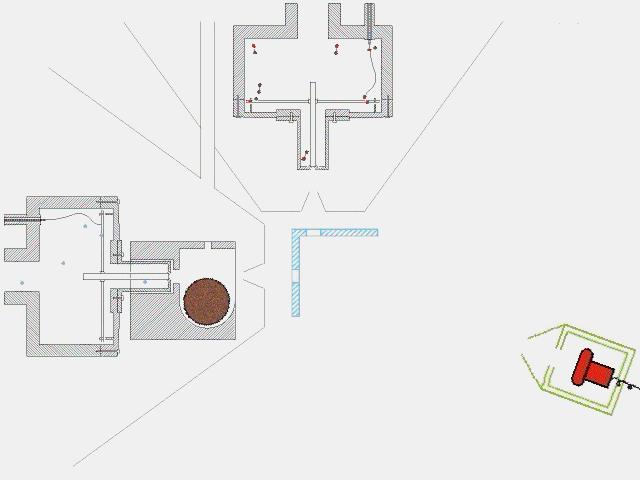
The timing of a typical experiment is shown in the adjacent animation (GIF). Normally we are working with carbon, dicarbon, tricarbon, boron, cyano, or ethynyl beams which are produced via laser ablation. Also, phenyl and allyl radicals have been prepared via flash pyrolysis. In addition, supersonic beams of ethynyl, vinyl, hydroxyl, and propargyl are generated via flash pyrolysis. In case of silicon, an ultraviolet laser pulse hits a rotating silicon rod to release silicon species which are subsequently seeded in helium or neon carrier gas released by the primary valve. This beam passes a skimmer, a chopper wheel (not shown in the animation for simplicity), and the cold shield to reach the interaction region, where it collides with the particles of a secondary pulsed beam. This secondary pulsed beam can be for example acetylene. As seen in the animation, a correct timing of the pulsed valves and the laser is necessary for the experiment to work. The products fly undisturbed in the direction of the detector and are analyzed at different angles in the time-of-flight mode, i.e. recording the intensity of an ion of a selected mass-to-charge ratio versus the time. The detector is shown here in a fast moving motion which is only to demonstrate its variable viewing angle.


| Source | Precursor | Generation |
|---|---|---|
| B(2P) | boron rod | laser ablation 266 nm |
| Al(2P) | aluminum rod | laser ablation 266 nm |
| C(3P) | graphite rod | laser ablation 266 nm |
| C(3P) | CHBr3 | photodissociation 248 nm |
| C(1D) | graphite rod | laser ablation 266 nm |
| C2(Χ1Σg+ / a3Πu) | graphite rod | laser ablation 266 nm |
| C2(Χ1Σg+ / a3Πu) | C2Cl4 | photodissociation 248 nm |
| C3(Χ1Σg+) | graphite rod | laser ablation 266 nm |
| Si(3P) | silicon rod | laser ablation 266 nm |
| Si(3P) | Si2H6 | photodissociation 193 nm |
| Si(1D) | Si2H6 | photodissociation 193 nm |
| Ge(3P) | germanium rod | laser ablation 266 nm |
| O(3P) | Al2O3 rod | laser ablation 266 nm |
| N(4S) | BN rod | laser ablation 266 nm |
| BO(Χ2Σ+) | boron rod / CO2 | laser ablation 266 nm & reaction |
| BS(Χ2Σ+) | boron rod / CS2 | laser ablation 266 nm & reaction |
| CN(Χ2Σ+) | graphite rod / N2 / N2O | laser ablation 266 nm & reaction |
| CD(Χ2Π) | graphite rod / D2 | laser ablation 266 nm & reaction |
| C2D(Χ2Σ+) | graphite rod / D2 | laser ablation 266 nm & reaction |
| SiN(Χ2Σ+) | silicon rod / N2 / N2O | laser ablation 266 nm & reaction |
| SiD(Χ2Π) | silicon rod / D2 | laser ablation 266 nm & reaction |
| SiH(Χ2Π) | Si2H6 | photolysis 193 nm |
| CH(Χ2Π) / CD(Χ2Π) | CHBr3 / CDBr3 | photolysis 248 nm |
| C2H(Χ2Σ+) | C2HBr | photolysis 193 nm |
| C2H3(Χ2Α′) | C2H3Br | photolysis 193 nm |
| CH3CC(Χ2Α1) | CH3CCI / CH3CCBr | photolysis 193 nm |
| C3H5(Χ2Α2) | C3H5I | pyrolysis |
| C6H5(Χ2Α1) | C6H5NO | pyrolysis |
| C6H5(Χ2Α1) | C6H5Cl | photolysis 193 nm |
| C7H7(Χ2Α1) / C7D7(Χ2Α1) (p) | C7H7Cl / C7D7Cl (p) | photolysis 193 nm |
| C7H7(Χ2Α′) / C7D7(Χ2Α′) (m) | C7H7Cl / C7D7Cl (m) | photolysis 193 nm |
| 2-C10H7(Χ2Α′) (2) | 2-C10H7Cl | photolysis 193 nm |
| C5H4N(Χ2Α′) (o/m) | C5H4NCl (o/m) | photolysis 193 nm |
| C4H | C4H2 | photolysis 193 nm |