
A Comprehensive Chemical Dynamics Study on the Decomposition Mechanism of Nitramine-/Nitro-Amine-based Energetic Materials and their Cocrystals in the Condensed Phase

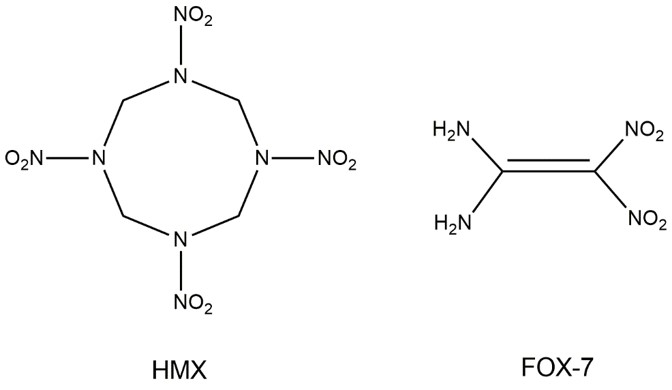
The primary goal of the proposal is to determine experimentally and computationally the fundamental reaction mechanisms, dynamics, and kinetics involved in the decomposition of a) nitramine-based energetic material (cyclotetramethylene tetranitramine, HMX), b) nitro-amine-based energetic material (1,1-diamino-2,2-dinitroethylene, FOX-7), and c) their cocrystal. Nitramine- and nitro-amine-based molecules such as HMX, FOX-7, RDX (1,3,5-trinitro-1,3,5-triazine), Cl-20 (hexanitro hexaazaisowurtzitane dodecane), and TATB (triaminotrinitrobenzene) feature high explosion efficiency (i.e. performance) that has promising potential in civilian and military applications. Nonetheless, their decomposition processes at the molecular level present a major challenge in the field of physical chemistry. This will be addressed in this project by a focused attack of combining emerging techniques in analytical photoionization and detection methodologies along with state-of-the-art methods in computational chemistry. To date, no comprehensive study has been conducted, in which the decomposition mechanisms of the nitramine- and nitro-amine-based energetic materials along with their cocrystals along with the overall spectrum of newly formed open- and closed-shell products are revealed on line and in situ in the condensed phase. The proposed research pushes the boundary of our knowledge on the interplay between the molecular structure of the energetic material and their behavior, such as performance and sensitivity, and will play a critical role in designing and synthesizing the next-generation energetic material either as a pure substance or cocrystals of high performance and low sensitivity.

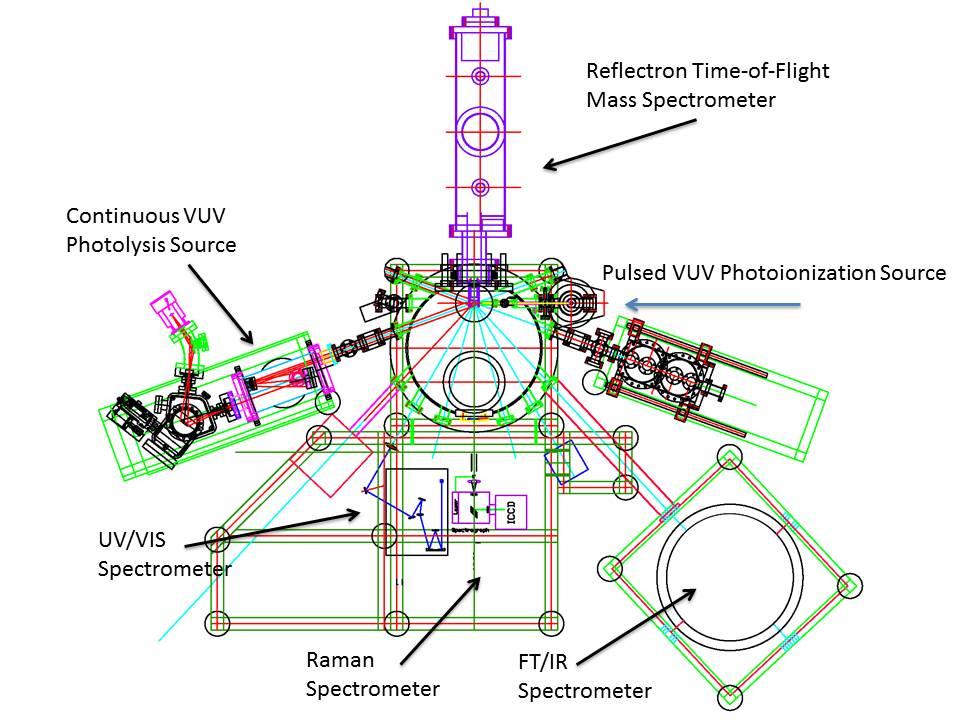
A tightly integrated collaboration spanning the full range from fundamental studies in reaction dynamics and kinetics along with electronic structure theory and quasiclassical trajectory simulations (Prof. Rui Sun, University of Hawaii) will be developed to achieve five specific objectives (O1-O5). Combining a unique instrumental design with innovative experiments (O1-O2) and pioneering quasiclassical dynamics simulations highlighting novel electronic structure calculations (O3-O4), the project provides a unified view and unprecedented information on the decomposition of energetic molecular materials in the condensed phase (O5). It highlights the first ever versatile concepts on the overall spectrum of newly formed open- and closed-shell products that drive the decomposition of molecular energetic materials as well as explore fundamental bond dissociation and formation processes on the molecular level. It is important to note, although the decomposition of energetic materials has been explored for over two decades as compiled in section III, previous studies were predominantly carried out in the gas phase. On the contrary, the proposed study reveals the fundamental, yet elusive such process at the molecular level in the solid state.
Recent Selected Publications
1. P. Maksyutenko, L.G Muzangwa, B.M. Jones, R.I. Kaiser, Lyman α Photolysis of Solid Nitromethane (CH3NO2) and D3-Nitromethane (CD3NO2) - Untangling the Reaction Mechanisms Involved in the Decomposition of Model Energetic Materials, Phys. Chem. Chem. Phys. 17, 7514 - 7527 (2015). (PDF)
2. R.I. Kaiser, P. Maksyutenko, A Mechanistical Study on Non-Equilibrium Reaction Pathways in Solid Nitromethane (CH3NO2) and D3-nitromethane (CD3NO2) upon Interaction with Ionizing Radiation, Chem. Phys. Lett. 631-632, 59-65 (2015). (PDF)
3. R.I. Kaiser, P. Maksyutenko, Novel Reaction Mechanisms Pathways in the Electron Induced Decomposition of Solid Nitromethane (CH3NO2) and D3- Nitromethane (CD3NO2), J. Phys. Chem. C 119, 14653-14668 (2015). (PDF)
4. M. Förstel, P. Maksyutenko, B.M. Jones, B-J Sun, S-H Chen, A.H.H. Chang, R.I. Kaiser, Detection of the Elusive Triazane Molecule (N3H5) in the Gas Phase, Chem. Phys. Chem. 16, 3139-3142 (2015). (PDF)
5. Y.A. Tsegaw, W. Sander, R.I. Kaiser, Electron Paramagnetic Resonance Spectroscopic Study on Nonequilibrium Reaction Pathways in the Photolysis of Solid Nitromethane (CH3NO2) and D3-Nitromethane (CD3NO2), J. Phys. Chem. A. 120, 1577-1587 (2016). (PDF)
6. P. Maksyutenko, M. Förstel, P. Crandall, B-J Sun, M.H. Wu, A.H.H. Chang, R.I. Kaiser, An isomer-specific study of solid nitromethane decomposition pathways - Detection of aci-nitromethane (H2CNO(OH)) and nitrosomethanol (HOCH2NO) intermediates, Chem. Phys. Lett., 658, 20-29 (2016). (PDF)
7. M. Förstel, Y.A. Tsegaw, P. Maksyutenko, A.M. Mebel, W. Sander, R.I. Kaiser, On the Formation of N3H3 Isomers in Irradiated Ammonia Bearing Ices: Triazene (H2NNNH) or Triimide (HNHNNH), Chem. Phys. Chem. 17, 2726-2735 (2016). (PDF)
8. S. Góbi, Parker B. Crandall, P. Maksyutenko, M. Förstel, R.I. Kaiser, Accessing the Nitromethane (CH3NO2) Potential Energy Surface in Methanol (CH3OH)–Nitrogen Monoxide (NO) Ices Exposed to Ionizing Radiation: An FTIR and PI-ReTOF-MS Investigation, J. Phys. Chem. A 122, 2329-2343 (2018). (PDF)
9. S. K. Singh, C. Zhu, V. Vuppuluri, S. F. Son, R. I. Kaiser, Probing the Reaction Mechanisms Involved in the Decomposition of Solid 1,3,5-Trinitro-1,3,5-triazinane by Energetic Electrons, J. Phys. Chem. A 123, 9479-9497 (2019). (PDF)
10. S. K. Singh, J. L. Jeunesse, V. Vuppuluri, S. F. Son, B. J. Sun, Y. L. Chen, A. H. H. Chang, A. M. Mebel, R. I. Kaiser The Elusive Ketene (H2CCO) Channel in the Infrared Multiphoton Dissociation of Solid 1,3,5-Trinitro-1,3,5-Triazinane (RDX), ChemPhysChem, 21, 837-842 (2020). (PDF)
11. S. K. Singh, T. Y. Tsai, B. J. Sun, A. H. H. Chang, A. M. Mebel, R. I. Kaiser, Gas Phase Identification of the Elusive N-Hydroxyoxaziridine (c-H2CON(OH)): A Chiral Molecule, J. Phys. Chem. Lett., 11, 5383-5389 (2020). (PDF)
12. S. K. Singh, V. Vuppuluri, S. F. Son, R. I. Kaiser, Investigating the Photochemical Decomposition of Solid 1,3,5-Trinitro-1,3,5-triazinane (RDX), J. Phys. Chem. A, 124, 6801-6823 (2020). (PDF)
13. S. K. Singh, J. L. Jeunesse, C. Zhu, N. F. Kleimeier, K. H. Chen, B. J. Sun, A. H. H. Chang, R. I. Kaiser, Gas Phase Identification of the Elusive Oxaziridine (cyclo-H2CONH) – An Optically Active Molecule , Chem. Commun., 56, 15643-15646 (2020). (PDF)
14. S. K. Singh, R. I. Kaiser, A Vacuum Ultraviolet Photoionization Study on the Isomerization, Decomposition, and Molecular Mass Growth Processes in Solid Nitromethane (CH3NO2), Chem. Phys. Lett., 766, 138343 (2021). (PDF)
15. S. K. Singh, V. Vuppuluri, B. J. Sun, B. Y. Chang, A. K. Eckhardt, S. F. Son, A. H. H. Chang and R. I. Kaiser, Identification of Elusive Keto and Enol Intermediates in the Photolysis of 1,3,5-Trinitro-1,3,5-Triazinane, J. Phys. Chem. Lett. 12, 6062-6069 (2021). (PDF)
16. A. M. Turner, Y. Luo, J. H. Marks, R. Sun, J. T. Lechner, T. M. Klapotke, R. I. Kaiser, Exploring the Photochemistry of Solid 1,1-Diamino-2,2-dinitroethylene (FOX-7) Spanning Simple Bond Ruptures, Nitro-to-Nitrite Isomerization, and Nonadiabatic Dynamics, J. Phys. Chem. A, 126, 29, 4747-4761 (2022). (PDF)
17. Y. Luo, C. Kang, R. I. Kaiser, R. Sun, The potential energy profile of the decomposition of 1,1-diamino-2,2-dinitroethylene (FOX-7) in the gas phase, Phys. Chem. Chem. Phys., 24, 26836-26847 (2022). (PDF) (Supplemental Information)
18. A. M. Turner, J. H. Marks, Y. Luo, J. T. Lechner, T. M. Klapotke, R. Sun, R. I. Kaiser, Electron-Induced Decomposition of Solid 1,1-Diamino-2,2-dinitroethylene (FOX-7) at Cryogenic Temperatures, J. Phys. Chem. A, 127, 3390-3401 (2023). (PDF) (Supplemental Information)
19. A. M Turner, J. H. Marks, J. T. Lechner, T. M. Klapotke, R. Sun, R. I. Kaiser, Ultraviolet-Initiated Decomposition of Solid 1,1-Diamino-2,2-dinitroethylene (FOX-7), J. Phys. Chem. A, 127, 37, 7707-7717 (2023). (PDF) (Supplemental Information)
20. K. Yadav, Y. Luo, R. I. Kaiser, R. Sun, Initial decomposition pathways of 1,1-diamino-2,2-dinitroethylene (a-FOX-7) in the condensed phase, Phys. Chem. Chem. Phys., 26, 11395-11405 (2024). (PDF) (Supplemental Information)
21. Y. Luo, K. Yadav, R. I. Kaiser, R. Sun, Theoretical Study of the Subsequent Decomposition Mechanisms of 1,1-Diamino-2,2-dinitroethene (FOX-7), J. Comp. Chem., 46, e27542 (2025). (PDF)

