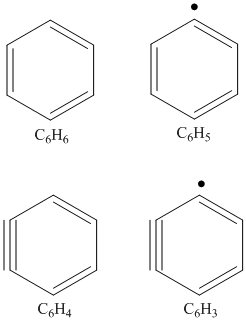
Our experiments exposed for the very first time that monocyclic aromatic molecules such as the phenyl radical (C6H5) and two prototypes of polycyclic aromatic hydrocarbons (PAHs), indene (C9H8) and naphthalene (C10H8), can be formed as a consequence of a single collision event in the gas phase. Exploiting the power of the crossed molecular beam approach, we investigated four key reaction classes relevant to the formation of polycyclic hydrocarbon molecules (PAHs) in combustion systems: i) the formation of resonantly stabilized free radicals (RSFRs), ii) the unimolecular decomposition of resonantly stabilized free radicals (RSFRs), iii) the formation of aromatic radicals (ARs), and iv) the reactions of the aromatic phenyl radical leading to polycyclic aromatic hydro-carbons (PAHs) together with their non-PAH isomers. Numerous projects were conducted in collaboration with experimental and theoretical groups participating in the DOE-BES Program including Musahid Ahmed (Lawrence Berkeley National Lab), Alexander Mebel (Florida International University), and William Green (MIT). Here, we investigated the formation of mono-cyclic aromatic molecules formed under single collision conditions in crossed molecular beam experiments via the reactions of 1,3-butadiene (C2H3C2H3) with ethynyl (C2H), of 1,3-butadiene (C2H3C2H3) with dicarbon (C2), of vinylacetylene (C2H3CCH) with ethynyl (C2H), and of vinyl-acetylene (C2H3CCH) with dicarbon (C2): benzene (C6H6), phenyl (C6H5), o-benzyne (C6H4), and 1,2,3-tridehydrobenzene (C6H3); note that the latter is only predicted to be formed from reaction with triplet dicarbon at levels of about 5%.
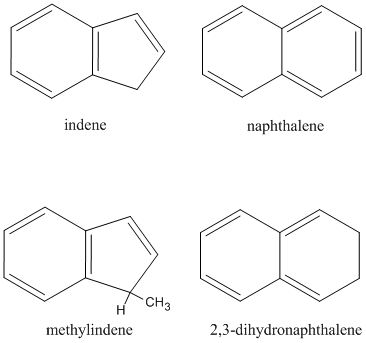
Chemical reaction networks modeling the formation of PAHs in combustion flames imply that the phenyl radical (C6H5) presents one of the most important transient species involved in the formation and complexation of PAHs. However, despite impressive kinetic data, the reaction products formed in bimolecular collisions of phenyl radicals with unsaturated hydrocarbons have remained elusive. Therefore, we engaged a systematic research program to investigate the reaction dynamics of phenyl radicals with unsaturated hydrocarbons under single collision conditions as provided in crossed molecular beam experiments over a wide range of collision energies from about 40 to 200 kJmol-1. Reactions at higher collision energies from 80 to 200 kJmol-1 tied up some lose ends from the previous funding period. Crossed beam experiments at lower collision energies focused on the reaction dynamics of phenyl radicals with hydrocarbon molecules accessing the C9Hx (x=8,10) and C10Hx (x = 6, 8,10) PESs and, hence, investigating the formation of bicyclic aromatic species holding the indene and naphthalene carbon skeletons. This was achieved by crossing supersonic beams of phenyl radicals with allene (H2CCCH2), methylace- tylene (CH3CCH), and propylene (CH3C2H3) (indene core) as well as diacetylene (HCCCCH), vinylacetylene (HCCC2H3), and 1,3-butadiene (C2H3C2H3) (naphthalene core). At lower collision energies of 40–50 kJmol-1, the crossed beam experiments of phenyl with methylacetylene (CH3CCH) and allene (H2CCCH2) as well as with vinylacetylene (C2H3CCH) depicted for the very first time that polycyclic aromatic hydrocarbons indene (C9H8) and naphthalene (C10H8) are formed as a result of a single collision event between the aromatic phenyl radical and a hydrogen-deficient hydrocarbon molecule. These lower collision energies lead to an enhanced life time of the initial collision complex allowing the latter to isomerize via hydrogen migration(s) and/or cyclization ultimately leading to PAH formation such as indene and naphthalene through hydrogen loss involving a tight exit transition state.
With respect to indene formation, our crossed molecular beam experiments of phenyl radicals with methylacetylene and allene as well as their (partially) deuterated counterparts provided compelling evidence that an individual polycyclic aromatic hydrocarbon can be formed as a result of a single collision event in the gas phase at a collision energy of about 45 kJmol-1. Considering the allene reaction, the C6H5H2CCCH2 isomer was identified as the central reaction intermediate formed either by addition of the phenyl radical to the C1 carbon atom of allene or via a one-step isomerization starting from a 2-phenylallyl intermediate formed by addition of the phenyl radical to the central carbon atom of allene. The C6H5H2CCCH2 intermediate isomerized via hydrogen migration(s) followed by cyclization(s) to the 3-hydro-indene intermediate. A unimolecular decomposition of the latter led via atomic hydrogen loss to indene. Most important, 2-phenylallyl is also accessed in the reaction of phenyl with methylacetylene involving a six-step isomerization sequence starting with the initial collision complex formed via addition of the phenyl radical to the acetylenic carbon atom of methylacetylene. On the other hand, the chemical dynamics involved in the formation of naphtha-lene under single collision conditions depict unique pattern. The reaction of the phenyl radical with vinylacetylene involved two low-energy pathways initiated by an addition of the phenyl radical to the terminal carbon atoms of the vinyl-acetylene reactant at the vinyl- and acetylene moieties. Most importantly, at the vinyl group, the addition involved the formation of a van-der-Waals complex stabilized by 19 kJmol-1 with respect to the separated reactants followed by an isomerization of the latter through a submerged barrier, whereas the competing addition at the acetylene group involves a barrier of 5 kJ mol-1. Both collision complexes ultimately isomerize to the 1-hydro-naphthyl intermediate, which emits atomic hydrogen to form naphthalene.

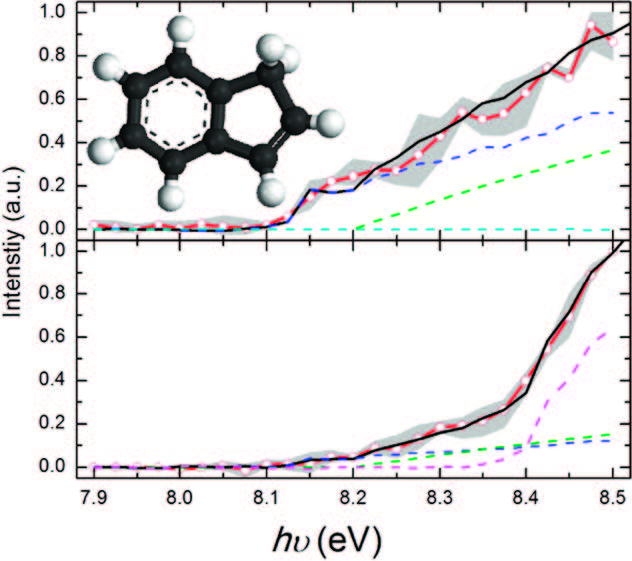
Complementary to the crossed beam experiments, we developed in collaboration with Musahid Ahmed (Lawrence Berkeley National Lab) an experimental protocol to investigate the formation mechanisms of PAHs within a high temperature chemical reactor by simulating the combustion relevant conditions. We successfully conduc-ted a directed synthesis of indene in situ in a continuous supersonic molecular beam through reaction of pyrolytically generated phenyl radicals (C6H5) with hydrocarbons (methylacetylene, allene) inside a heated silicon carbide tube (chemical reactor). Indene together with its acyclic isomers were then photoionized by vacuum ultraviolet (VUV) light from the Advanced Light Source at the Chemical Dynamics Beamline at various photon energies from 7.5 to 12.0 eV to record photoionization efficiency (PIE) curves. Based on known PIE curves of the PAH and their acyclic isomers, the recorded PIE curves were then simulated theoretically to extract the nature of the products formed and their branching ratios. Reaction mechanisms and branching ratios are explained in terms of new electronic structure calculations in collaboration with Alexander Mebel (Florida International University). Prior to this study, no experiment has been conducted in which an individual PAH was formed via a gas phase reaction under well-defined conditions in a high temperature chemical reactor. The data analysis of the phenyl/propylene system is in progress.
Resonantly stabilized free radicals (RSFRs) such as the propargyl (C3H3;X2B3) radical are believed to play a crucial role in the formation of polycyclic aromatic hydrocarbons (PAHs) and soot, in the combustion of fuels, and in the chemical processing of carbon rich circumstellar envelopes. Due to the delocalization of the unpaired electron, resonantly stabilized free hydrocarbon radicals are more stable than ordinary radicals and form weaker bonds with stable molecules such as molecular oxygen in combustion flames. These weakly bound complexes are not easily stabilized by collisions at high temperatures. Therefore, RSFRs are relatively unreactive and can reach high concentration in flames. These high concentrations and the relatively fast rates of the corresponding radical – radical reactions make them important in the mechanism of formation of complex hydrocarbons such as the very first aromatic ring species in flames and in the interstellar medium. For instance, Miller and Melius as well as Kern and Xie suggested odd-carbon-atom reaction pathways involving the recombination of two propargyl radicals. Electronic structure calculations imply that the initially formed acyclic collision complex(es) of the recombination of two propargyl radicals can isomerize via multiple steps to ultimately form benzene and/or the phenyl radical plus a hydrogen atom. An alternative even-odd-carbon-atom sequence could include the reaction of propargyl radicals (C3H3) with acetylene (C2H2) to yield the cyclopentadienyl radical (C5H5). The latter can react in multiple steps to benzene.
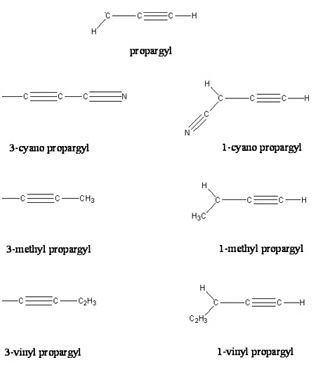
Nevertheless, reactions of substituted propargyl radicals, which in turn could yield substituted benzene molecules and cyclopentadienyl radicals via reaction with a propargyl radical and acetylene molecule, respectively, have not been included yet in reaction models simulating hydrocarbon flames and the chemistry in circumstellar envelopes such as of IRC+10216. For instance, the cyano propargyl radical, i.e. a propargyl radical in which a hydrogen at the C1 or C3 position is replaced by a cyano (CN) group, could react with propargyl and acetylene to form cyano benzene (C6H5CN) and cyano cyclopentadienyl radicals (C5H4CN), respectively. However, to validate this proposed route, the first step is to elucidate possible formation routes to the cyano propargyl radical itself. A recent crossed beams study on the reaction of ground state carbon atoms, C(3Pj), with ethylene, C2H4(X1A1), suggested that the propargyl radical and atomic hydrogen are the dominating reaction products. Therefore, a reaction of carbon atoms with vinyl cyanide (cyano ethylene; C2H3CN(X1A’)), i.e. a substituted ethylene molecule, is expected to yield cyano propargyl radicals under single collision conditions. Very recently, Su et al. investigated this reaction computationally. The authors concluded that cyano propargyl radicals are the sole reaction products under single collision conditions. However, an experimental verification of these computations remains to be carried out.
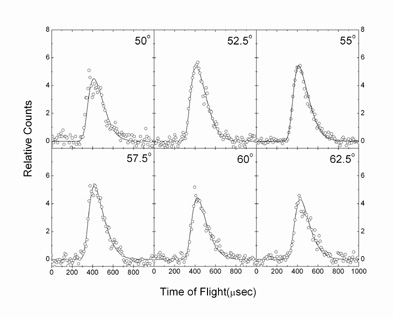
Here, the chemical dynamics of the reaction of ground state carbon atoms, C(3Pj), with vinyl cyanide, C2H3CN(X1A’), were examined under single collision conditions at collision energies of 29.9 kJmol-1 and 43.9 kJmol-1 using the crossed molecular beams approach. Our investigations suggest that the reaction follows indirect scattering dynamics via addition of the carbon atom to the carbon-carbon double bond of the vinyl cyanide molecule yielding a cyano cyclopropylidene collision complex. The latter undergoes ring opening to form cis/trans triplet cyano allene which fragments predominantly to the 1-cyano propargyl radical via tight exit transition states; the 3-cyano propargyl isomer was inferred to be formed at least a factor of two less. The discovery of the cyano propargyl radical in the reaction of atomic carbon with vinyl cyanide under single collision conditions implies that this molecule can be an important reaction intermediate in combustion flames and also in extraterrestrial environments (cold molecular clouds, circumstellar envelopes of carbon stars) which could lead to the formation of cyano benzene (C6H5CN) upon reaction with a propargyl radical.
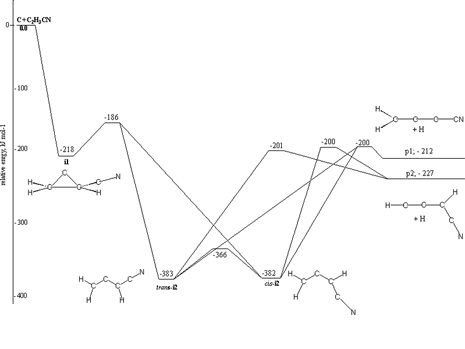

It is interesting to compare the present findings with the reactions of ethylene, propylene, and 1,3-butadiene studied recently using crossed molecular beams. Here, the reaction dynamics and potential energy surfaces involved in the formation of (substituted) propargyl radicals show common features. All reactions are dictated by indirect scattering dynamics; about 30 – 35% of the total available energy channels into the translational degrees of freedom. The carbon atom adds to the π electron density. This yields (substituted) cyclopropylidene intermediates which are stabilized by 210 – 220 kJmol-1 with respect to the reactants. These cyclic intermediates undergo ring opening through barriers of between 30 and 50 kJmol-1 to form (substituted) cis/trans allene intermediates on the triplet surface. The latter are bound by 340 – 380 kJmol-1 compared to the separated reactants. The fate of the triplet allene complexes is governed by hydrogen atom loss channels to (substituted) propargyl radicals via exit transition states located about 10 – 35 kJmol-1 above the products. Most of the initial angular momentum channels into rotational excitation of the products. The existence of an exit barrier is documented in the center-of-mass translation energy distributions; here, all P(ET)s peak around 20 – 50 kJmol-1. Finally, the exoergicities to form the C3H2R (R = CH3, C2H3, H, CN) products are very similar and fall between 190 and 220 kJmol-1; in all reactions investigated, the 1-substituted propargyl radical was found to be formed systematically in higher yields compared to the 3-substituted isomer with ratios of 3:1 (propylene), 8:1 (1,3-butadiene), and 5:1 (vinyl cyanide).
Recent Selected Publications
1. R.I. Kaiser, A.G. Suits, A High-Intensity, Pulsed Supersonic Carbon Source with C(3Pj) Kinetic Energies of 0.08 - 0.7 eV for Crossed Beam Experiments, Rev. Sci. Instrum. 66, 5405-5411 (1995). (PDF)
2. R.I. Kaiser, Y.T. Lee, A.G. Suits, Crossed Beam Reaction of C(3Pj) with C2H2(1Σg+): Observation of Tricarbon-Hydride C3H, J. Chem. Phys. 103,10395-10398 (1995). (PDF)
3. R.I. Kaiser, Y.T. Lee, A.G. Suits, Crossed-beam reaction of carbon atoms with hydrocarbon molecules. I. Chemical dynamics of the propargyl radical formation, C3H3 (X2B2), from reaction of C(3Pj) with ethylene, C2H4 (X1Ag), J.Chem. Phys. 105, 8705-8720 (1996). (PDF)
4. R.I. Kaiser, D. Stranges, Y.T. Lee, A.G. Suits, Crossed-beam reaction of carbon atoms with hydrocarbon molecules. II. Chemical dynamics of n-C4H3 formation from reaction of C(3Pj) with methylacetylene, CH3CCH (X1A1), J. Chem. Phys. 105, 8721-8733 (1996). (PDF)
5. R.I. Kaiser, C. Ochsenfeld, M. Head-Gordon, Y.T. Lee, A.G. Suits, A combined experimental and theoretical study on the formation of interstellar C3H isomers, Science 274, 1508-1511 (1996). (PDF)
6. R.I. Kaiser, C. Ochsenfeld, M. Head-Gordon, Y.T. Lee, A.G. Suits, Crossed-beam reaction of carbon atoms with hydrocarbon molecules. III: Chemical dynamics of propynylidyne (l-C3H; X2) and cyclopropynylidyne (c-C3H; X2B2) formation from reaction of C(3Pj) with acetylene, C2H2(X1Σg+), J. Chem. Phys. 106, 1729-1741 (1997). (PDF)
7. R.I. Kaiser, D. Stranges, Y.T. Lee, A.G. Suits, Neutral-Neutral reactions in the interstellar medium. I. formation of carbon hydride radicals via reaction of carbon atoms with unsaturated hydrocarbons, Ap. J. 477, 982-989 (1997). (PDF)
8. R.I. Kaiser, Y.T. Lee, A.G. Suits, M. Head-Gordon, A Coupled-Cluster ab initio study of triplet C3H2 and the neutral-neutral reaction to interstellar C3H, J. Chem. Phys. 106, 4141-4151 (1997). (PDF)
9. R.I. Kaiser, D. Stranges, H.M. Bevsek, Y.T. Lee, A.G. Suits, Crossed-beam reaction of carbon atoms with hydrocarbon molecules. IV. Chemical dynamics of methylpropargyl radical formation, C4H5, from reaction of C(3Pj) with propylene, C3H6 (X1A'), J. Chem. Phys. 106, 4945-4953 (1997). (PDF)
10. R.I. Kaiser, W. Sun, A. G. Suits, Crossed beam reaction of atomic carbon C(3Pj) with hydrogen sulfide, H2S (X1A1): observation of the thioformyl radical, HCS(X2A'), J. Chem. Phys. 106, 5288-5291 (1997). (PDF)
11. R.I. Kaiser, W. Sun, A.G. Suits, Y.T. Lee, Crossed beam reaction of atomic carbon, C(3Pj), with the propargyl radical, C3H3(X2B2): Observation of diacetylene, C4H2(X1Σg+), J. Chem. Phys. 107, 8713-8716 (1997). (PDF)
12. R.I. Kaiser, C. Ochsenfeld, M. Head-Gordon, Y.T. Lee, The formation of HCS and HCSH Molecules and Their Role in the Collision of Comet Shoemaker-Levy 9 with Jupiter, Science 279, 1181-1184 (1998). (PDF)
13. H. Habara, S. Yamamoto, C. Ochsenfeld, M. Head-Gordon, R.I. Kaiser, Y.T. Lee, Fourier transform millimeter-wave spectroscopy of the HCS radical in the 2A' ground electronic state, J. Chem. Phys. 108, 8859-8863 (1998). (PDF)
14. R.I. Kaiser, C. Ochsenfeld, D. Stranges, M. Head-Gordon, Y.T. Lee, Combined Crossed Molecular Beams and ab initio Investigation of the Formation of Carbon-bearing Molecules in the interstellar medium via neutral-neutral reactions, Farad. Discussion 109, 183-204 (1998). (PDF)
15. R.I. Kaiser et al., General Discussion, Faraday Discussion 109, 83-108 (1998). (PDF)
16. R.I. Kaiser et al., General Discussion, Faraday Discussion 109, 217-256 (1998). (PDF)
17. R.I. Kaiser et al., General Discussion, Faraday Discussion 109, 493-516 (1998). (PDF)
18. R.I. Kaiser, C. Ochsenfeld, M. Head-Gordon, Y.T. Lee, Neutral-Neutral reactions in the interstellar medium. II. isotope effects in the formation of linear and cyclic C3H and C3D radicals in interstellar environments, Ap. J. 510 784-788 (1999). (PDF)
19. R.I. Kaiser, C. Ochsenfeld, M. Head-Gordon, Y.T. Lee, Crossed-beam reaction of carbon atoms with sulfur containing molecules. I. Chemical dynamics of thioformyl (HCS X2A') formation from reaction of C(3Pj) with hydrogen sulfide, H2S(X1A1), J. Chem. Phys.110, 2391-2403 (1999). (PDF)
20. R.I. Kaiser, I. Hahndorf, L.C.L. Huang, Y.T. Lee, H.F. Bettinger, P.v.R. Schleyer, H.F. Schaefer III, P.R. Schreiner, Crossed beams reaction of atomic carbon, C(3Pj), with d6-benzene, C6D6(X1A1g): Observation of the per-deutero-1,2-didehydro-cycloheptatrienyl radical, C7D5(X2B2), J. Chem. Phys. 110, 6091-6094 (1999). (PDF)
21. C. Ochsenfeld, R.I. Kaiser, Y.T. Lee, M. Head-Gordon, Coupled-Cluster ab initio investigation of singlet/triplet CH2S isomers and the reaction of atomic carbon with hydrogen sulfide to HCS/HSC, J. Chem. Phys 110, 9982-9988 (1999). (PDF)
22. R.I. Kaiser, A.M. Mebel, A.H.H. Chang, S.H. Lin, Y.T. Lee, Crossed-beam reaction of carbon atoms with hydrocarbon molecules. V. Chemical dynamics of n-C4H3 formation from reaction of C(3Pj) with allene, H2CCCH2 (X1A1), J. Chem. Phys. 110, 10330 - 10344 (1999). (PDF)
23. A.M. Mebel, R.I. Kaiser, Y.T. Lee, Ab initio MO study of the global potential energy surface of C4H4 in triplet electronic state and the reactions of C(3Pj) with C3H4 (allene and propyne) and C2(A3Пu) with C2H4(X1A1g+), J. Am. Chem. Soc. 122, 1776-1788 (2000). (PDF)
24. R.I. Kaiser, O. Asvany, Y.T. Lee, H.F. Bettinger, P.v.R. Schleyer, H.F. Schaefer III, Crossed beam reaction of phenyl radicals with unsaturated hydrocarbon molecules. I. Chemical dynamics of phenylmethylacetylene (C6H5CCCH3; X1A') formation from reaction of C6H5(X2A1) with methylacetylene, CH3CCH(X1A1,), J. Chem. Phys 112, 4994-5001 (2000). (PDF)
25. R.I. Kaiser, O. Asvany, Y.T. Lee, Crossed beam investigation of elementary reactions relevant to the formation of polycyclic aromatic hydrocarbon (PAH)-like molecules in extraterrestrial environments, Planetary and Space Science 48,483-492 (2000). (PDF)
26. R.I. Kaiser, N. Balucani, O. Asvany, Y.T. Lee, Astrochemistry: From molecular clouds to Planetary systems, Y.C.Minh, E.F. van Dishoeck (eds.), Crossed molecular beam experiments of radical-neutral reactions relevant to the formation of hydrogen deficient molecules in extraterrestrial environments, IAU Series 197, 251-264 (2000).
27. H.F. Bettinger, P.v.R. Schleyer, H.F. Schaefer III, P.R. Schreiner, R.I. Kaiser,Y.T. Lee, The reaction of benzene with a ground state carbon atom, C(3Pj), J. Chem. Phys. 113, 4250-4263 (2000). (PDF)
28. I. Hahndorf, H.Y. Lee, A.M. Mebel,S.H. Lin, Y.T. Lee, R.I. Kaiser, A combined crossedbeam and ab initio investigation on the reaction of carbonspecies with C4H6 isomers. I. the 1 ,3-butadiene molecule, H2CCHCHCH2 (X1A'), J. Chem. Phys. 113, 9622-9636 (2000). (PDF)
29. L.C.L. Huang, H.Y. Lee, A.M. Mebel, S.H. Lin, Y.T. Lee, R.I. Kaiser, A combined crossed beam and ab initio investigation on the reaction of carbon species with C4H6 isomers. II. The dimethylacetylene molecule, H3CCCCH3 (X1A1g), J. Chem. Phys. 113, 9637-9648 (2000). (PDF)
30. R.I. Kaiser, A. Mebel, Y.T. Lee, Chemical dynamics of cyclopropynylidyne (c-C3H; X2B2) formation from the reaction of C(1D) with acetylene, C2H2(X1Σg+), J. Chem. Phys. 114, 231-239 (2001). (PDF)
31. R.I. Kaiser, H.Y. Lee, A.M. Mebel, Y. T. Lee, The formation of C5H5 isomers as potential key intermediates to polycyclic aromatic hydrocarbon-like molecules, Ap.J. 548, 852-860 (2001). (PDF)
32. T.N. Lee, H.Y. Lee, A.M. Mebel, R.I. Kaiser, Ab initio MO study of the triplet C3H4 potential energy surface and the reaction of C(3Pj) with ethylene, C2H4, J. Phys. Chem. A. 105, 1847-1856 (2001). (PDF)
33. T.L. Nguyen, A.M. Mebel, R.I. Kaiser, A theoretical investigation of the triplet carbon atom C(3P) + vinyl radical C2H3(2A') reaction and thermochemistry of C3Hn (n=1-4) species, J. Phys. Chem. A 105, 3284-3299 (2001). (PDF)
34. C.C. Chiong, O. Asvany, N. Balucani, Y.T. Lee, R.I. Kaiser, Nucleation of hydrogen deficient carbon clusters in circumstellar envelops of carbon stars, World Scientific Press, Proceedings of the 8th Asia-Pacific Physics Conference, APPC 2000, 167-169 (2001).
35. T.N. Le, A.M. Mebel, R.I. Kaiser, Ab Initio Study of the C4H3 Potential Energy Surface and reaction of ground-state carbon atom with propargyl radical, J. Comp. Chem. (special issue in honor of Prof. Schleyers 70th birthday), 22, 1522-1535 (2001). (PDF)
36. N. Balucani, H.Y. Lee, A.M. Mebel, Y.T. Lee, R.I. Kaiser, A combined crossed beam and ab initio investigation on the reaction of carbon species with C4H6 isomers. III. 1,2-butadiene, H2CCCH(CH3) (X1A') - a non-Rice-Ramsperger-Kassel-Marcus system?, J. Chem. Phys., 115, 5107-5116 (2001). (PDF)
37. R.I. Kaiser, A.M. Mebel, Y.T. Lee, A.H.H. Chang, Unimolecular decomposition of chemically activated triplet C4HD3 complexes: a combined crossed-beam and ab initio study, J. Chem. Phys., 115, 5117-5125 (2001). (PDF)
38. N. Balucani, A. M. Mebel, Y.T. Lee, R.I. Kaiser, A Combined Crossed Molecular Beam and Ab Initio Study of the Reactions C2(X1Σg+,a3Πu) + C2H4 -> n-C4H3(X2A') + H(2S1/2), J. Phys. Chem. A, 43, 9813-9818 (2001). (PDF)
39. R.I. Kaiser, T.L. Lee, T.L. Nguyen, A.M. Mebel, N. Balucani, Y.T. Lee, F. Stahl, P.v.R. Schleyer, H.F. Schaefer III, A combined crossed molecular beam and ab initio investigation of C2 and C3 elementary reactions with unsaturated hydrocarbons - pathways to hydrogen deficient hydrocarbon radicals in combustion flames, Faraday Discussion 119, 51-66 (2001). (PDF)
40. R.I. Kaiser et al. General Discussion, Faraday Discussions 119, 121-143 (2001). (PDF)
41. R.I. Kaiser et al. General Discussion, Faraday Discussions 119, 255-274 (2001). (PDF)
42. R.I. Kaiser et al. General Discussion, Faraday Discussions 119, 353-370 (2001). (PDF)
43. R.I. Kaiser, T.L. Nguyen, T.N. Le, A.M. Mebel, An ab initio investigation of reactions of carbon atoms, C(3Pj), with C2H4 and C3H6 in the interstellar medium, Ap.J. 561, 858-863 (2001). (PDF)
44. T.L. Nguyen, A.M. Mebel, S.H. Lin, R.I. Kaiser, Product branching ratios of the C(3P) + C2H3(2A')and CH(2Π) + C2H2 (1Σg+) reactions and photodissociation of H2CC=CH(2B1) at 193 and 242 nm: An ab initio/RRKM study, J. Phys. Chem. A, 43, 9813-9818 (2001). (PDF)
45. R.I. Kaiser, Y.T. Lee, Laboratory chemical dynamics in hydrocarbon rich atmospheres of outer planets, Highlights of Astronomy, Astronomical Society of the Pacific, 12, 88-91 (2002).
46. A.M. Mebel, R.I. Kaiser, The formation of interstellar C2N isomers in circumstellar envelopes of carbon stars: an ab initio study, Ap.J. 564, 787-791 (2002). (PDF)
47. R.I. Kaiser, T.L. Nguyen, A.M. Mebel, Y.T. Lee, Stripping dynamics in the reactions of electronically excited carbon atoms, C(1D), with ethylene and propylene - production of propargyl and methylpropargyl radicals, J. Chem. Phys. 116, 1318-1324 (2002). (PDF)
48. I. Hahndorf, Y.T. Lee, R.I. Kaiser, L. Vereecken, J. Peeters, H.F. Bettinger, P.R. Schreiner, P.v.R. Schleyer, W.D. Allen, H.F. Schaefer III, A combined crossed-beam, ab initio, and Rice-Ramsperger-Kassel-Marcus investigation of the reaction of carbon atoms C(3Pj) with benzene, C6H6(X1A1g) and d6-benzene, C6D6(X1A1g), J. Chem. Phys. 116, 3248-3262 (2002). (PDF)
49. L. Vereecken, J. Peeters, H.F. Bettinger, R.I. Kaiser, P.v.R. Schleyer, H.F. Schaefer III, Reaction of phenyl radicals with propyne, JACS 124, 2781-2789 (2002). (PDF)
50. R.I. Kaiser, Experimental Investigation on the Formation of Carbon-Bearing Molecules in the Interstellar Medium via Neutral-Neutral Reactions, Chem. Rev., 102, 1309-1358 (2002). (PDF)
51. R.I. Kaiser, M. Yamada, Y. Osamura, A crossed beam and ab initio investigation of the reaction of hydrogen sulfide, H2S(X1A1), with dicarbon molecules, C2(X1 Σg+), J. Phys. Chem. A, 106, 4825-4832 (2002). (PDF)
52. R.I. Kaiser, A.M. Mebel, The reactivity of ground-state carbon atoms with unsaturated hydrocarbons in combustion flames and in the interstellar medium, Int. Rev. in Physical Chemistry, 21, 307-356 (2002). (PDF)
53. A.M. Mebel, R.I. Kaiser, An ab initio Study on the Formation of Interstellar Tricarbon Isomers 1-C3(X1 Σg+) and c-C3(X3A'2), Chem. Phys. Lett., 360, 139-143 (2002). (PDF)
54. M. Yamada, Y. Osamura, R.I. Kaiser, A comprehensive investigation on the formation of organo-sulfur molecules in dark clouds via neutral-neutral reactions, Astronomy and Apics 395, 1031-1044 (2002). (PDF)
55. T. L. Nguyen, A. M. Mebel, R. I. Kaiser, Potential Energy Surface and Product Branching Ratios for the Reaction of C(3Pj) with the Allyl Radical: An Ab Initio/RRKM Study, J. Phys. Chem. A, 107, 2990-2999 (2003). (PDF)
56. R.I. Kaiser, L. Vereecken, J. Peeters, H.F. Bettinger, P.v.R. Schleyer, H.F. Schaefer III, Elementary reactions of the phenyl radical, C6H5, with C3H4 isomers, and of benzene, C6H6, with atomic carbon in extraterrestrial environments, Astronomy & Apics, 406, 385-391 (2003). (PDF)
57. R.I. Kaiser, N. Balucani, D.O. Charkin, A.M. Mebel, A crossed beam and ab initio study of the C2(X1Σg+/a3Πu) + C2H2(X1 Σg+) reactions, Chem. Phys. Lett. 382, 112-119 (2003). (PDF)
58. H.F. Su, R.I. Kaiser, A.H.H. Chang, A theoretical study for the reaction of vinyl cyanide C2H3CN(X1A') with the ground state carbon atoms C(3P) in cold molecular clouds, J. Chem. Phys. 122, 074320/1-20 (2005). (PDF)
59. H.Y. Li, W.C. Cheng, Y.L. Liu, B.J. Sun, C.Y. Huang, K.T. Chen, M.S. Tang, R.I. Kaiser, A. H. H. Chang, Reaction of cyanoacetylene HCCCN(X1Σ+) with ground-state carbon atoms C(3P) in cold molecular clouds, J. Chem. Phys, 124, 44307-1-19 (2006). (PDF)
60. A.M. Mebel, V.V. Kislov, R.I. Kaiser, Potential Energy Surface and Product Branching Ratios for the Reaction of Dicarbon,C2(X1 Σg+), with Methylacetylene, CH3CCH(X1A1): An Ab Initio/RRKM Study, JPCA 110, 2421-2433 (2006). (PDF)
61. Y. Guo, X. Gu, R.I. Kaiser, Mass Spectrum of the 1-Butene-3-yne-2-yl Radical (i-C4H3; X2A'), Int. J. Mass Spectrometry 249-250, 420-425 (2006). (PDF)
62. X. Gu, Y. Guo, E. Kawamura, R.I. Kaiser, Characteristics and Diagnostics of a Ultrahigh Vacuum Compatible Laser Ablation Source for Crossed Molecular Beam Experiments, J. Vac. Science & Technology A, 24, 505-511 (2006). (PDF)
63. Y. Guo, X. Gu, N. Balucani, R.I. Kaiser, Formation of the 2,4-pentadiynyl-1 radical (H2CCCCCH, X2B1) in the Crossed Beams Reaction of Dicarbon Molecules with Methylacetylene, JPCA 110, 6245-6249 (2006). (PDF)
64. X. Gu, Y. Guo, A.M. Mebel, R.I. Kaiser, Chemical Dynamics of the Formation of the 1,3-Butadiynyl Radical (C4H(X2 Σ+)) and its Isotopomers, JPCA, 110, 11265-11278 (2006). (PDF)
65. Y. Guo, X. Gu, F. Zhang, A.M. Mebel, R.I. Kaiser, Unimolecular Decomposition of Chemically Activated Pentatetraene (H2CCCCCH2) Intermediates - A Crossed Beams Study of Dicarbon Molecule Reactions with Allene, JPCA 110, 10699-10707 (2006). (PDF)
66. X. Gu, Y. Guo, F. Zhang, A.M. Mebel, R.I. Kaiser, Reaction Dynamics of Carbon-Bearing Radicals in Circumstellar Envelopes of Carbon Stars, Faraday Discussion 133: Chemical Evolution of the Universe, 245-275 (2006). (PDF)
67. A. M. Mebel, V. V. Kislov, R. I. Kaiser, Ab Initio/RRKM Study of the Singlet C4H4 Potential Energy Surface and of the Reactions of C2(X1 Σg+) with C2H4(X1A1g) and C(1D) with C3H4 (Allene and Methylacetylene), J. Chem. Phys. 125, 133113-1/15(2006). (PDF)
68. Y. Guo, X. Gu, F. Zhang, A.M. Mebel, R. I. Kaiser, Reaction Dynamics of Small Carbon Clusters with Unsaturated Hydrocarbons in the Interstellar Medium, in: Astrochemistry - From Laboratory Studies to Observations. American Institute of Physics, 855, 42-54 (2006).
69. C.S. Jamieson, Y. Guo, X. Gu, F. Zhang, C.J. Bennett, R.I. Kaiser, Laboratory Studies on the Formation of Carbon-Bearing Molecules in Extraterrestrial Environments - From the Gas Phase to the Solid State, Proceedings of the NASA Laboratory Astrophysics Workshop; NASA Special Issue, CP - 2006, 214549, 68-71 (2006).
70. Y. Guo, X. Gu, F. Zhang, A.H.H. Chang, R.I. Kaiser, A Crossed Beams Study of the Reaction of Carbon Atoms, C(3Pj), with Vinyl Cyanide, C2H3CN(X1A') - Investigating the Formation of Cyano Propargyl Radicals, Phys. Chem. Chem. Phys, 8, 5454 - 5461 (2006). (PDF)
71. X. Gu,Y. Guo, F. Zhang, A.M. Mebel, R.I. Kaiser, A Crossed Molecular Beams Study of the Reaction of Dicarbon Molecules with Benzene, Chem. Phys. Lett 436, 7-14 (2007). (PDF)
72. X. Gu,Y. Guo, R.I. Kaiser, Mass Spectra of the 2,4-Pentadiynylidyne (C5H; X2П) and the 2,4-Pentadiynyl-1 (n-C5H3; X2B1) Radicals, Int. J. Mass Spectrometry 261, 100-107 (2007). (PDF)
73. X. Gu,Y. Guo, F. Zhang, R.I. Kaiser, Investigating the Chemical Dynamics of the Reaction of Ground-State Carbon Atoms with Acetylene and Its Isotopomers, JPCA. 111, 2980-2992 (2007). (PDF)
74. Y. Guo, X. Gu, F. Zhang, B-J Sun, M.F. Tsai, A.H.H. Chang, R.I. Kaiser, Unraveling the Formation of HCPH (X2A') Molecules in Extraterrestrial Environments: Crossed Molecular Beam Study of the Reaction of Carbon Atoms, C(3Pj), with Phosphine, PH3(X1A1), JPCA. 111, 3241-3247 (2007). (PDF)
75. Y. Guo, X. Gu, F. Zhang, A.M. Mebel, R.I. Kaiser, A Crossed Molecular Beam Study on the Formation of Hexenediynyl Radicals (H2CCCCCCH; C6H3 (X2A')) via Reactions of Tricarbon Molecules, C3(X1 Σg+), with Allene (H2CCCH2; X1A1) and Methylacetylene (CH3CCH; X1A1), Phys. Chem. Chem. Phys, 9, 1972-1979 (2007). (PDF)
76. R.I. Kaiser, L. Belau, S.R. Leone, M. Ahmed, Y. Wang, B.J. Braams, J. Bowman, A Combined Experimental and Computational Study on the Ionization Energies of the Cyclic and Linear C3H Isomers, Chem. Phys. Chem. 8, 1236-1239 (2007). (PDF)
77. Y. Guo, A.M. Mebel, F. Zhang, X. Gu, R.I. Kaiser, Crossed Molecular Beam Studies of the Reactions of Allyl Radicals, C3H5(X2A2) with Methylacetylene (CH3CCH(X1A1)), Allene (H2CCCH2(X1A1)), and Their Isotopomers, JPCA 111, 4914-4921 (2007). (PDF)
78. X. Gu, Y. Guo, F. Zhang, A.M. Mebel, R.I. Kaiser, A Crossed Molecular Beams Study on the Formation and Energetics of the Resonantly Stabilized Free i-C4H3(X2A1) radical, and its Isotopomers, Chemical Physics 335, 95-108 (2007). (PDF)
79. A.M. Mebel, G.S. Kim, V.V. Kislov, R.I. Kaiser, The Reaction of Tricarbon with Acetylene: An Ab Initio/RRKM Study of the Potential Energy Surface and Product Branching Ratios, JPCA 111, 6704-6712 (2007). (PDF)
80. X. Gu, Y. Guo, F. Zhang, A.M. Mebel, R.I. Kaiser, Unimolecular Decomposition of Chemically Activated Singlet and Triplet D3-Methyldiacetylene Molecules, Chem. Phys. Lett. 444, 220-225 (2007). (PDF)
81. X. Gu, F. Zhang, Y. Guo, R.I. Kaiser, A Crossed Molecular Beam Study on the Formation of Phenylacetylene from Phenyl Radicals and Acetylene, Angew. Chem. Int. Ed., 46, 6866-6869 (2007). (PDF)
82. F. Zhang, X. Gu, Y. Guo, R.I. Kaiser, Reaction Dynamics on the Formation of Styrene: A Crossed Molecular Beam Study of the Reaction of Phenyl Radicals with Ethylene, J. Organic Chem. 72, 7597-7604 (2007). (PDF)
83. X. Gu, F. Zhang, R.I. Kaiser, A Crossed Beam Reaction of the Phenyl Radical, (C6H5, X2A1) with Molecular Oxygen (O2; X3Σg-): Observation of the Phenoxy Radical, (C6H5O, X2A'), Chem. Phys. Lett. 448, 7-10 (2007). (PDF)
84. X. Gu, F. Zhang, Y. Guo, R.I. Kaiser, Reaction Dynamics of Phenyl Radicals (C6H5, X2A') with Methylacetylene (CH3CCH(X1A1)), Allene (H2CCCH2(X1A1)), and Their D4-Isotopomers, J. Phys. Chem. A 111, 11450-11459 (2007). (PDF)
85. A.M. Mebel, V. V. Kislov, R.I. Kaiser, Theoretical Studies of Potential Energy Surfaces and Product Branching Ratios for the Reactions of C2 with Small Unsaturated Hydrocarbons (Acetylene, Ethylene, Methylacetylene, and Allene), Gas Phase Molecular Reaction and Photodissociation Dynamics, Editors: K.C. Lin and P.D. Kleiber, Transworld Research Network, pp. 113-159 (2007). (PDF)
86. X. Gu, Y. Guo, A.M. Mebel, R.I. Kaiser, A Crossed Beams Investigation of the Reactions of Tricarbon Molecules, C3(X1Σg+), with Acetylene, C2H2(X1Σg+), Ethylene, C2H4(X1Ag), and Benzene C6H6(X1A1g), Chem. Phys. Lett. 449, 44-52 (2007). (PDF)
87. X. Gu, R.I. Kaiser, A.M. Mebel, Chemistry of Energetically Activated Cumulenes - From Allene (H2CCCH2) to Hexapentaene (H2CCCCCCH2), Invited Review. Chem. Phys. Chem. 9, 350-369 (2008). (PDF)
88. F. Zhang, X. Gu, R.I. Kaiser, Formation of the Diphenyl Molecule in the Crossed Beam Reaction of Phenyl Radicals with Benzene, J. Chem. Phys. 128, 084315/1-5 (2008). (PDF)
89. F. Zhang, X. Gu, Y. Guo, R.I. Kaiser, Reaction Dynamics of Phenyl Radicals (C6H5) with Propylene (CH3CHCH2) and Its Deuterated Isotopologues, JPCA 112, 3284-3290 (2008). (PDF)
90. C.Y. Huang, B-J. Sun, H.H. Kuo, K.T. Chen, H.L. Sun, C.H. Huang, M.F. Tsai, C.H. Kao, Y.S. Wang, L.G. Gao, R.I. Kaiser, Formation of Interstellar 4-Pentadiynylidene,HCCCCC(X2Π), via the Neutral-Neutral Reaction of Ground State Carbon, C(3P) with Diacetylene, HCCCCH(X1Σg+), JCP 128, 244303/1-16 (2008). (PDF)
91. F. Zhang, Y.S. Kim, L. Zhou, A.H.H. Chang, R.I. Kaiser, Directed Gas Phase Synthesis of the Interstellar 2,4-Pentadiynylidyne Radical (HCCCCC), JCP 129, 134313 (2008). (PDF)
92. X. Gu, F. Zhang, R.I. Kaiser, A Crossed Molecular Beam Study of the Phenyl Radical Reaction with 1,3-Butadiene and its Deuterated Isotopomers, JPCA 113, 998-1006 (2009). (PDF)
93. F. Zhang, Y.S. Kim, R.I. Kaiser, A.M. Mebel, On the Formation of the 1,3,5-Hexatriynyl Radical (C6H(X2Π)) via the Crossed Beams Reaction of Dicarbon (C2(X1Σg+/a3Πu)), with Diacetylene (C4H2(X1Σg+)), JPCA 113, 1210-1217 (2009). (PDF)
94. X. Gu, R.I. Kaiser, Reaction Dynamics of Phenyl Radicals in Extreme Environments: A Crossed Molecular Beam Study, Acc. Chem. Res. 42, 290-302 (2009). (PDF)
95. X. Gu, F Zhang, R.I. Kaiser, V.V. Kislov, A.M. Mebel, Reaction Dynamics of the Phenyl Radical with 1,2-Butadiene, CPL 474, 51-56 (2009). (PDF)
96. J. Sun, C.H. Huang,, M.F. Tsai, H.L. Sun, L.G. Gao, Y.S. Wang, Y.Y. Yeh, Y.H. Shih, Z.F. Sia, P.H. Chen, R.I. Kaiser, A.H.H. Chang, Synthesis of Interstellar 1,3,5-Heptatriynylidyne, C7H(X2Π), via the Neutral-Neutral Reaction of Ground State Carbon Atom, C(3P) with Triacetylene, HC6H.(X1Σg+), JCP 131, 104305/1-13 (2009). (PDF)
97. R.I. Kaiser, F. Zhang, X. Gu, V.V. Kislov, A.M. Mebel, Reaction Dynamics of the Phenyl Radical (C6H5) with 1-Butyne (HCCC2H5) and 2-Butyne (CH3CCCH3), CPL 481, 46-53 (2009). (PDF)
98. R.I. Kaiser, A.M. Mebel, O. Kostko, M. Ahmed. On the Ionization Energies of C4H3 Isomers, CPL 485, 281-285 (2010). (PDF)
99. R.I. Kaiser, Reaction Dynamics of Carbon-Centered Radicals in Extreme Environments Studied by the Crossed Molecular Beams Technique, Carbon-Centered Radicals: Structure, Dynamics and Reactivity. Malcolm D. E. Forbes, Editor, Wiley, p. 221-248 (2010).
100. P. Maksyutenko, F. Zhang, X. Gu, R.I. Kaiser, A Crossed Molecular Beam Study on the Reaction of Methylidyne Radicals [CH(X2Π)] with Acetylene [C2H2(X1Σg+)] - Competing C3H2 + H and C3H + H2 Channels, PCCP 13, 240-252 (2011). (PDF)
101. A. Landera, R.I. Kaiser, A.M. Mebel, Addition of One and Two Units of C2H to Styrene: A Theoretical Study of the C10H9 and C12H9 Systems and Implications towards Growth of Polycyclic Aromatic Hydrocarbons at Low Temperatures, JCP 134, 024302/1-13 (2011). (PDF)
102. F. Zhang, D.S.N. Parker, Y.S. Kim, R.I. Kaiser, A.M. Mebel, On the Formation of Ortho-benzyne (o-C6H4) Under Single Collision Conditions and its Role in Interstellar Chemistry, Ap. J. 728:141, 1-10 (2011). (PDF)
103. D.S.N. Parker, F. Zhang, Y.S. Kim, R.I. Kaiser, A.M. Mebel, On the Formation of Resonantly Stabilized C5H3 Radicals - A Crossed Beam and Ab Initio Study of the Reaction of Ground State Carbon Atoms with Vinylacetylene, JPCA 115, 593-601 (2011). (PDF)
104. F. Zhang, R.I. Kaiser, V.V. Kislov, A.M. Mebel, A. Golan, M. Ahmed, A VUV Photoionization Study of the Formation of the Indene Molecule and Its Isomers, J. Phys. Chem. Lett., 2, 1731-1735 (2011). (PDF)
105. R.I. Kaiser, M. Goswami, P. Maksyutenko, F. Zhang, Y.S. Kim, A. Landera, A.M. Mebel, A Crossed Molecular Beams and Abm Initio Study on the Formation of C6H3 Radicals. An Interface between Resonantly Stabilized and Aromatic Radicals, J. Phys. Chem. A., 115, 10251-10258 (2011). (PDF)
106. D.S.N. Parker, F. Zhang, R.I. Kaiser, Phenoxy Radical (C6H5O) Formation under Single Collision Conditions from Reaction of the Phenyl Radical (C6H5, X2A1) with Molecular Oxygen (O2, X3Σg-): The Final Chapter?, J. Phys. Chem. A, 115, 11515-11518 (2011). (PDF)
107. D.S.N. Parker, F. Zhang, R.I. Kaiser, V. Kislov, A.M. Mebel, Indene Formation under Single-Collision Conditions from Reaction of Phenyl Radicals with Allene and Methylacetylene - A Crossed Molecular Beam and Ab Initio Study, Chem. Asian J., 6, 3035-3042 (2011). (PDF)
108. A.V. Wilson, D.S.N. Parker, F. Zhang, R.I. Kaiser, Crossed Beam Study of the Atom-Radical Reaction of Ground State Carbon Atoms (C(3P)) with the Vinyl Radical (C2H3(X2A')), Phys. Chem. Chem. Phys, 14, 477-481 (2012). (PDF)
109. F. Zhang, P. Maksyuntenko, R.I. Kaiser, Chemical Dynamics of the CH(X2Π)] + C2H4(X1A1g), CH(X2Π) + C2D4(X1A1g), and CD(X2Π) + C2H4(X1A1g) Reactions Studied Under Single Collision Conditions, Phys. Chem. Chem. Phys, 14, 529-537 (2012). (PDF)
110. R.I. Kaiser, X. Gu, F. Zhang, P. Maksyuntenko, Crossed Beam Reaction of Methylidyne [CH(X2Π)] with D2-Acetylene [C2D2(X1Σg+)] and D1-Methylidyne [CD(X2Π)] with Acetylene [C2D2(X1Σg+)], Phys. Chem. Chem. Phys, 14, 575-588 (2012). (PDF)
111. R.I. Kaiser, D.S.N. Parker, M. Goswami, F. Zhang, V.V. Kislov, A.M. Mebel, J. Aguilera-Iparraguirre, W.H. Green, Crossed Beam Reaction of Phenyl and D5-Phenyl Radicals with Propene and Deuterated Counterparts-Competing Atomic Hydrogen and Methyl Loss Pathways, Phys. Chem. Chem. Phys, 14, 720-729 (2012). (PDF)
112. D.S.N. Parker, F. Zhang, Y.S. Kim, R.I. Kaiser, A. Landera, A.M. Mebel, On the Formation of Phenyldiacetylene (C6H5CCCCH) and D5-Phenyldiacetylene (C6D5CCCCH) Studied Under Single Collision Conditions, Phys. Chem. Chem. Phys, 14, 2997-3003 (2012). (PDF)
113. F. Zhang, R.I. Kaiser, A. Golan, M. Ahmed, H. Hansen, A VUV Photoionization Study of the Combustion-Relevant Reaction of the Phenyl Radical (C6H5) with Propylene (C3H6) in a High Temperature Chemical Reactor, J. Phys. Chem. A, 116, 3541-3546 (2012). (PDF)
114. R.I. Kaiser, D.S.N. Parker, F. Zhang, A. Landera, V.V. Kislov, A.M. Mebel, PAH Formation under Single Collision Conditions: Reaction of Phenyl Radical and 1,3-Butadiene to Form 1,4-Dihydronaphthalene, J. Phys. Chem. A, 116, 4248-4258 (2012). (PDF)
115. A. Golan, M. Ahmed, A.M. Mebel, R.I. Kaiser, A VUV Photoionization Study on the Multichannel Reaction of Phenyl Radicals with 1,3-butadiene Under Combustion Relevant Conditions, Phys. Chem. Chem. Phys., 15, 341-347 (2013). (PDF)
116. B.B. Dangi, S. Maity, R.I. Kaiser, A.M. Mebel, A Combined Crossed Beam and Ab Initio Investigation of the Gas Phase Reaction of Dicarbon Molecules (C2; X1Σg+/a3Πu) with Propene (C3H6; X1A'): Identification of the Resonantly Stabilized Free Radicals 1- and 3-Vinylpropargyl, J. Phys. Chem. A., 117, 11783-11793 (2013). (PDF)
117. D.S.N. Parker, T. Yang, R.I. Kaiser, A. Landera, A.M. Mebel, On the formation of ethynylbiphenyl (C14D5H5; C6D5C6H4CCH) isomers in the reaction of D5-phenyl radicals (C6D5; X2A1) with phenylacetylene (C6H5C2H; X1A1) under single collision conditions, CPL, 595-596, 230-236 (2014). (PDF)
118. D.S.N. Parker, B.B. Dangi, R.I. Kaiser, A. Jamal, M.N. Ryazantsev, K. Morokuma, A. Korte, W. Sander, An Experimental and Theoretical Study on the Formation of 2-Methylnaphthalene (C11H10 /C11H3D7) in the Reactions of the Para-Tolyl (C7H7) and Para-Tolyl-d7 (C7D7) with Vinylacetylene (C4H4), J. Phys. Chem. A, 118, 2709-2718 (2014). (PDF)
119. D.S.N. Parker, S. Maity, B.B. Dangi, R.I. Kaiser, A. Landera, A.M. Mebel, Understanding the chemical dynamics of the reactions of dicarbon with 1-butyne, 2-butyne, and 1,2-butadiene - toward the formation of resonantly stabilized free radicals, Phys. Chem. Chem. Phys., 16, 12150-12163 (2014). (PDF)
120. B.B. Dangi, D.S.N. Parker, R.I. Kaiser, D. Belisario-Lara, A.M. Mebel, An experimental and theoretical investigation of the formation of C7H7 isomers in the bimolecular reaction of dicarbon molecules with 1,3-pentadiene, Chem. Phys. Lett., 607, 92-99 (2014). (PDF)
121. T. Yang, D.S.N. Parker, B.B. Dangi, R.I. Kaiser, V.V. Kislov, A.M. Mebel, Crossed Beam Reactions of the Phenyl (C6H5; X2A1) and Phenyl-d5 Radical (C6D5; X2A1) with 1,2-Butadiene (H2CCCHCH3; X1A'), JPCA, 118, 4372-4381 (2014). (PDF)
122. B.B. Dangi, T. Yang, R.I. Kaiser, A.M. Mebel, Reaction Dynamics of the 4-Methylphenyl Radical (C6H4CH3; p-tolyl) with Isoprene (C5H8) - Formation of Dimethyldihydronaphthalenes, Phys. Chem. Chem. Phys., 16, 16805-16814 (2014). (PDF)
123. R.I. Kaiser, B.B. Dangi, T. Yang, D.S.N. Parker, A.M. Mebel, Reaction Dynamics of the 4-Methylphenyl Radical (p-Tolyl) with 1,2-Butadiene (1-Methylallene): Are Methyl Groups Purely Spectators?, JPCA, 118, 6181-6190 (2014). (PDF)
124. D.S.N. Parker, B.B. Dangi, R.I. Kaiser, A. Jamal, M. Ryazantsev and K. Morokuma, Formation of 6-Methyl-1,4-dihydronaphthalene in the Reaction of the p-Tolyl Radical with 1,3-Butadiene under Single-Collision Conditions, J. Phys. Chem. A, 118, 12111-12119 (2014). (PDF)