A Spectroscopic Study of the Reaction Mechanisms of the Oxidation of Metal-Doped exo-Tetrahydrodicyclopentadiene (JP-10) in Levitated Droplets
The primary objective of this project is to unravel the fundamental reaction mechanisms and kinetics involved in the oxidation of JP-10 (exo-tetrahydrodicyclopentadiene) jet fuel, doped with prototype classes of metal-based additives [pre-stressed aluminum (Al), α-aluminum hydride (AlH3), and reactive metal nanopowders (RMNP; Ti-Al, Ti-B, Ti-Al-B)], in the presence of molecular oxygen (O2). These findings will be exploited to develop high-energy-density fuel materials for air breathing ramjet/scramjet applications and as a component in liquid/gel bipropellant rockets. By combining these results with electronic structure calculations, the proposed investigations i) untangle the initial bond breaking and formation processes involving the oxidizer, hydrocarbon, and metal, ii) identify the nature of the primary reaction intermediates, iii) explore the successive isomerization processes of these intermediates, iv) advance the fundamental understanding of the oxidation processes on the most fundamental, microscopic level, v) transform the knowledge of the unimolecular decomposition of chemically activated reaction intermediates and evaluate the processes which control them (energy transfer), vi) establish predictive concepts of (non-adiabatic) reaction mechanisms in metal-doped jet fuel triggered thermally (infrared photons), and vii) illuminate the temperature-and pressure dependent chemical kinetics of the oxidation processes and reveal how metal additives influence and possibly catalyze the underlying reaction mechanism (transition states, overall activation energy). These findings push the boundaries of traditional theory and experimentation revealing energy concepts that are beyond conventional chemistry hence overcoming previous limitations of metal-mediated combustion processes such as the formation of oxide layers.

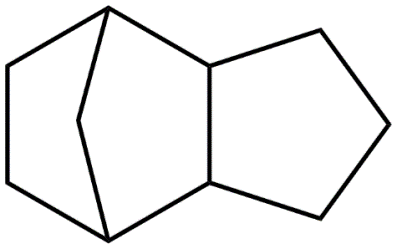

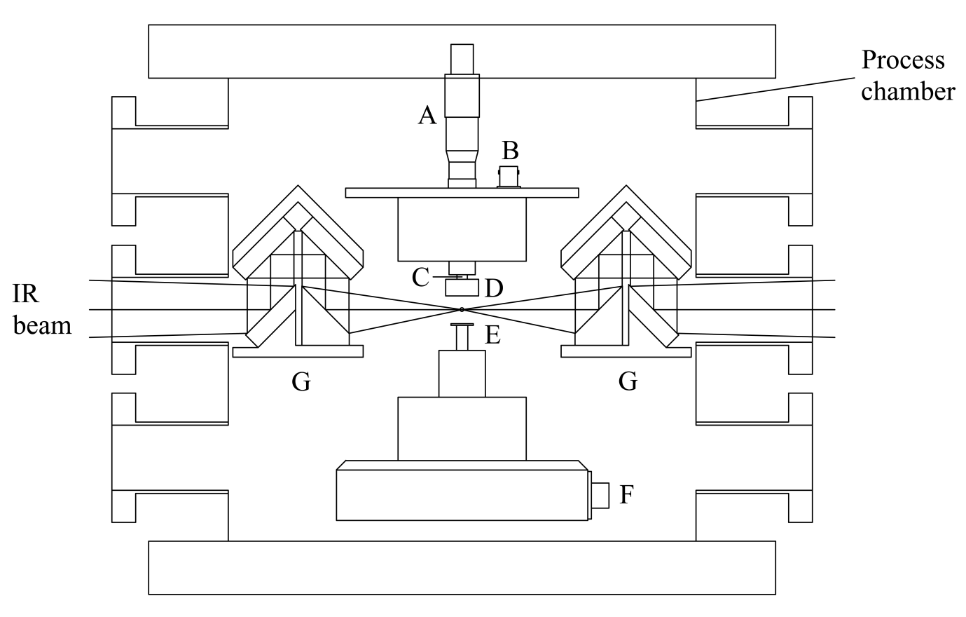
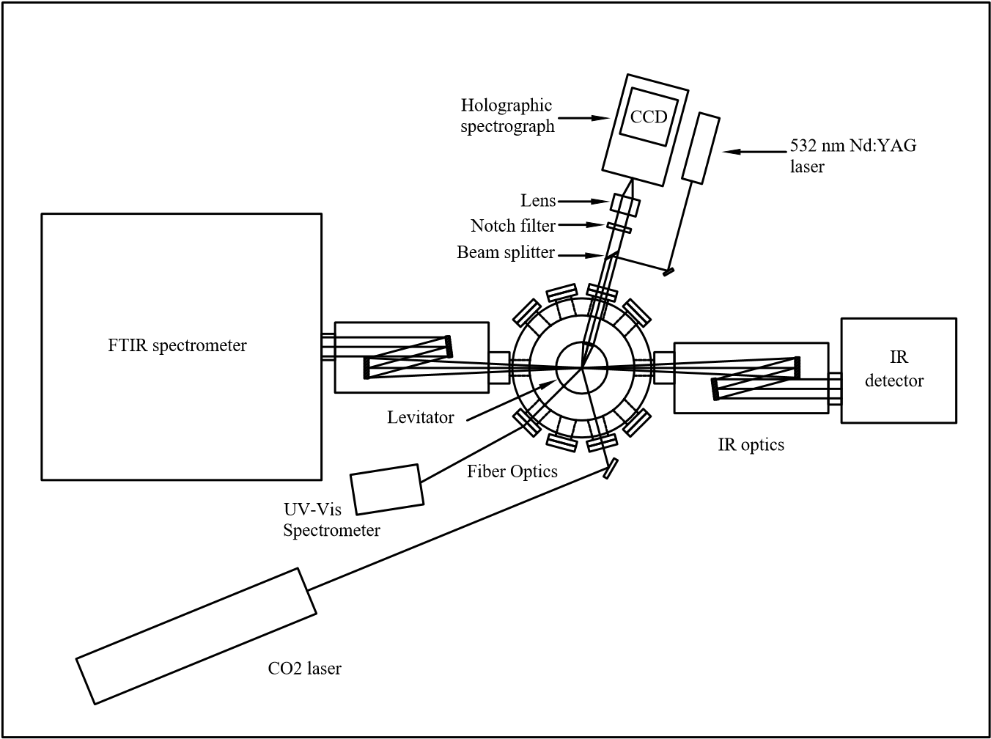
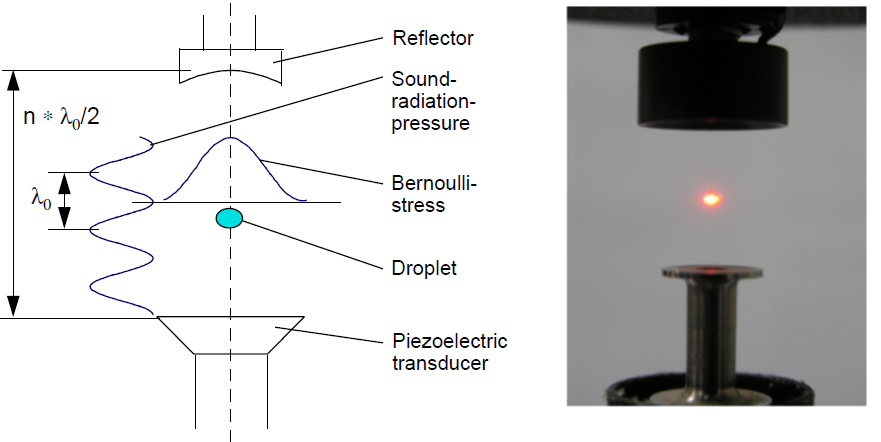
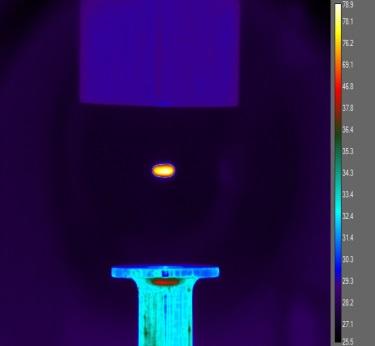
These objectives are achieved by systematically initiating the reaction and ignition of metal-doped JP-10 in levitated droplets under container-less conditions employing a novel ultrasonic levitation device at combustion-relevant temperatures and pressures. The levitation device represents an emerging laboratory technique, which has not been accessible in previous studies probing the oxidation of JP-10 in the presence of metal additives. To identify the products formed in these processes online and in situ, the levitator incorporates three highly complementary detection schemes eventually revealing the complex reactions and ignition processes of metal-doped JP-10 liquids comprehensively: infrared (IR), Raman (Ra), and ultraviolet – visible (UV-VIS) spectroscopy. By conducting these experiments with levitated droplets of metal- bearing JP-10 and tracing the temporal evolution of the reactants and products on line and in situ spectroscopically, we extract versatile concepts on the decomposition and reaction mechanisms along with the kinetics and products formed in these processes. These data are of crucial significance to understand metal-enhanced combustion processes, the efficiency, and ultimately energy releases of JP-10.
Besides the basic scientific interest from the physical chemistry community (reaction mechanisms) to unravel the decomposition and reaction pathways of metal-doped JP-10 jet fuel in levitated droplets, the studies have unprecedented implications to the fields of metal-supported combustion chemistry, air-breathing chemical propulsion systems, and also to the physical-organic chemistry community to unravel fundamental decomposition mechanisms of complex organic molecules and potential catalytic effects of the metal, to probe isomerization processes of organic transient species, to probe distinct fragmentation mechanisms of organics, and to advance insights into basic and chemical bonding of carbon-, hydrogen-, oxygen-, and metal-bearing molecules formed in these processes. This presents a major challenge with fundamental relevance to the Office of Naval Research to provide enhancements to key diagnostic capabilities of metal-based energetic material additives to jet fuel thus delivering fundamental insights into the combustion and potentially into the phenomena surrounding ignition and growth of energetic material additive to hydrocarbon fuel. This research is conducted in collaboration with Albert Epshteyn (US Naval Research Laboratory), Michelle Pantoya (Texas Tech University), Igor Schweigert (Naval Research Lab), and Greg Young (Naval Surface Warfare Center).



Recent Selected Publications
1. S.J. Brotton, and R.I. Kaiser, In Situ Raman Spectroscopic Study of Gypsum (CaSO4 ⋅ 2H2O) and Epsomite (MgSO4 ⋅ 7H2O) Dehydration Utilizing an Ultrasonic Levitator, JPCL, 4, 669-673 (2013). (PDF)
2. S.J. Brotton, R.I. Kaiser, Novel high-temperature and pressure-compatible ultrasonic levitator apparatus coupled to Raman and Fourier transform infrared spectrometers, Rev. Sci. Instrum. 84, 055114 (2013). (PDF)
3. S. Góbi, A. Bergantini, A.M. Turner, R.I. Kaiser, Electron Radiolysis of Ammonium Perchlorate: A Reflectron Time-of-Flight Mass Spectrometric Study, J. Phys. Chem. A 121, 3879-3890 (2017). (PDF)
4. S. Góbi, L. Zhao, B. Xu, U. Ablikim, M. Ahmed, R.I. Kaiser, A vacuum ultraviolet photoionization study on the thermal decomposition of ammonium perchlorate, Chem. Phys. Lett., 691, 250-257 (2018). (PDF)
5. S.J. Brotton, M. Lucas, S.D. Chambreau, G.L. Vaghjiani, J. Yu, S.L. Anderson, R.I. Kaiser, Spectroscopic Investigation of the Primary Reaction Intermediates in the Oxidation of Levitated Droplets of Energetic Ionic Liquids, J. Phys. Chem. Lett., 8, 6053-6059 (2018). (PDF)
6. S.J. Brotton, M. Lucas, T.N. Jensen, S.L. Anderson, R.I. Kaiser, Spectroscopic Study on the Intermediates and Reaction Rates in the Oxidation of Levitated Droplets of Energetic Ionic Liquids by Nitrogen Dioxide, J. Phys. Chem. A 122, 7351-7377 (2018). (PDF)
7. M. Lucas, S.J. Brotton, S.K. Shukla, J. Yu, S.L. Anderson, R.I. Kaiser, Oxidation of a Levitated Droplet of 1-Allyl-3-methylimidazolium Dicyanamide by Nitrogen Dioxide, J. Phys. Chem. A 123, 400-416, (2019). (PDF)
8. M. Lucas, S.J. Brotton, J.A.P. Sprenger, M. Finze, S.K. Sharma, R.I. Kaiser, Oxidation of a Levitated 1-Butyl-3-methylimidazolium Dicyanoborate Droplet by Nitrogen Dioxide, J. Phys. Chem. A 123, 780-795 (2019). (PDF)
9. S. J. Brotton, R. I. Kaiser, Spectroscopic Study on the Polymer Condensates Formed via Pyrolysis of Levitated Droplets of Dicyanamide-Containing Ionic Liquids, J. Phys. Chem. A 123, 1153−1167 (2019). (PDF) (Supplemental Information)
10. M. Lucas, S.J. Brotton, A. Min, M.L. Pantoya, R.I. Kaiser Oxidation of Levitated exo-Tetrahydrodicyclopentadiene Droplets Doped With Aluminum Nanoparticles, J. Phys.Chem. Lett., 10, 5756-5763 (2019). (PDF) (Supplemental Information)
11. M. Lucas, S. J. Brotton, A. Min, C. Woodruff, M. L. Pantoya, and R. I. Kaiser, Effects of Size and Prestressing of Aluminum Particles on the Oxidation of Levitated exo-Tetrahydrodicyclopentadiene Droplets, J. Phys.Chem. A., 124, 1489-1507 (2020). (PDF) (Supplemental Information)
12. S. J. Brotton and R. I. Kaiser, Controlled Chemistry via Contactless Manipulation and Merging of Droplets in an Acoustic Levitator, Anal. Chem, 92, 8371-8377 (2020). (PDF) (Supplemental Information)
13. S. J. Brotton, M. J. Malek, S. L. Anderson, R. I. Kaiser, Effects of acetonitrile-assisted ball-milled aluminum nanoparticles on the ignition of acoustically levitated exo-tetrahydrodicyclopentadiene (JP-10) droplets, Chem. Phys. Lett. 754, 137679 (2020). (PDF) (Supplemental Information)
14. L. Zhao, W. Y. Chen, H. J. Su, J. Z. Yang, R. I. Kaiser A vacuum ultraviolet photoionization study on oxidation of JP-10 (exo-Tetrahydrodicyclopentadiene), Chem. Phys. Lett. 754, 137490 (2020). (PDF) (Supplemental Information)
15. S. J. Brotton, R. I. Kaiser Effects of Nitrogen Dioxide on the Oxidation of Levitated exo-Tetra-hydro-dicyclopentadiene (JP-10) Droplets Doped with Aluminum Nanoparticles, J. Phys. Chem. A., 125, 2727-2742 (2021). (PDF) (Supplemental Information)
16. S. D. Perera, S. J. Brotton, H. Shinsato, R. I. Kaiser, Y. Choi, K. Na, Catalytic Effects of Zeolite Socony Mobil-5 (ZSM-5) on the Oxidation of Acoustically Levitated exo-Tetrahydrodicyclopentadiene (JP-10) Droplets, J. Phys. Chem. A, 125, 4896-4909 (2021). (PDF) (Supplemental Information)
17. S. J. Brotton, S.D. Perera, A. Misra, N. F. Kleimeier, A.M. Turner, R.I. Kaiser, M. Palenik, M. T. Finn, A. Epshteyn, B.-J. Sun, A.H.H. Chang, A Spectroscopic Investigation on the Oxidation of exo-Tetrahydrodicyclopentadiene (JP-10; C10H16) Doped with Titanium-Aluminum-Boron Reactive Metal Nanopowder (RMNP), J. Phys. Chem. A. , 126, 1, 125-144 (2022). (PDF) (Supplemental Information)
18. I. Antonov, A. Chyba, S. D. Perea, A. M. Turner, M. L. Pantoya, M. T. Finn, A. Epshteyn, R. I. Kaiser, Discovery of Discrete Stages in the Oxidation of exo-Tetrahydrodicyclopentadiene (C10H16) Droplets Doped with Titanium–Aluminum–Boron Reactive Mixed-Metal Nanopowder, J. Chem. Phys. Lett., 13, 9777-9785 (2022). (PDF) (Supplemental Information)
19. G. L. Rizzo, S. Biswas, I. Antonov, K. K. Miller, M. L. Pantoya, R. I. Kaiser, Exotic Inverse Kinetic Isotopic Effect in the Thermal Decomposition of Levitated Aluminum Iodate Hexahydrate Particles, J. Phys. Chem. Lett., 14, 2722–2730 (2023). (PDF) (Supplemental Information)
20. S. Biswas, D. Paul, C. He, N. Dias, M. Ahmed, M. L. Pantoya, R. I. Kaiser, Counterintuitive Catalytic Reactivity of the Aluminum Oxide “Passivation” Shell of Aluminum Nanoparticles Facilitating the Thermal Decomposition of exo-Tetrahydrodicyclopentadiene (JP-10), J. Phys. Chem. Lett., 14, 9341−9350 (2023). (PDF) (Supplemental Information)
21. S. Biswas, D. Paul, N. Dias, W. Lu, M. Ahmed, M. L. Pantoya, R. I. Kaiser, Efficient Oxidative Decomposition of Jet-Fuel exo-Tetrahydrodicyclopentadiene (JP-10) by Aluminum Nanoparticles in a Catalytic Microreactor: An Online Vacuum Ultraviolet Photoionization Study, J. Phys. Chem. A, 128, 1665−1684 (2024). (PDF) (Supplemental Information)
22. G. L. Rizzo, S. Biswas, M. L. Pantoya, R. I. Kaiser, Unraveling the Ignition Chemistry of Singly Levitated Aluminum Iodate Hexahydrate (AIH) Particles, Chem. Phys. Lett., 842, 141212 (2024). (PDF) (Supplemental Information)
23. S. Biswas, D. Paul, N. Dias, K. Kunzler, M. Ahmed, M. L. Pantoya, R. I. Kaiser, Stress-Alteration Enhancement of the Reactivity of Aluminum Nanoparticles in the Catalytic Decomposition of exo-Tetrahydrodicyclopentadiene (JP-10), J. Phys. Chem. A, 128, 3613−3624 (2024). (PDF) (Supplemental Information)
24. D. Paul, S. Biswas, H. Yeom, K. Na, M. L. Pantoya, R. I. Kaiser, Unraveling the Nanosheet Zeolite-Catalyzed Combustion of Aluminum Nanoparticles-Doped exo-Tetrahydrodicyclopentadiene (JP-10) Energetic Fuel, ACS Appl. Mater. Interfaces, 16, 53938−53949 (2024). (PDF) (Supplemental Information)
25. G. L. Rizzo, S. Biswas, D. Ka, X. Zheng, R. I. Kaiser, Untangling the Efficient Boron-Initialized Hydroxyl-Terminated Polybutadiene Combustion for High Energetic Solid Propulsion Systems, J. Phys. Chem. A, 129, 288-300 (2025). (PDF) (Supplemental Information)
26. S. Biswas, J. Cokas, W. Gee, D. Paul, N. Dias, H. W. T. Morgan, M. T. Finn, B. M. Hudak, P. M. Godbold, C. A. Klug, A. Epshteyn, A. N. Alexandrova, M. Ahmed R. I. Kaiser, Unconventional low temperature decomposition of a saturated hydrocarbon over atomically-dispersed titanium-aluminum-boron catalyst, Nat. Comm., 16, 6793 (2025). (PDF) (Supplemental Information)

