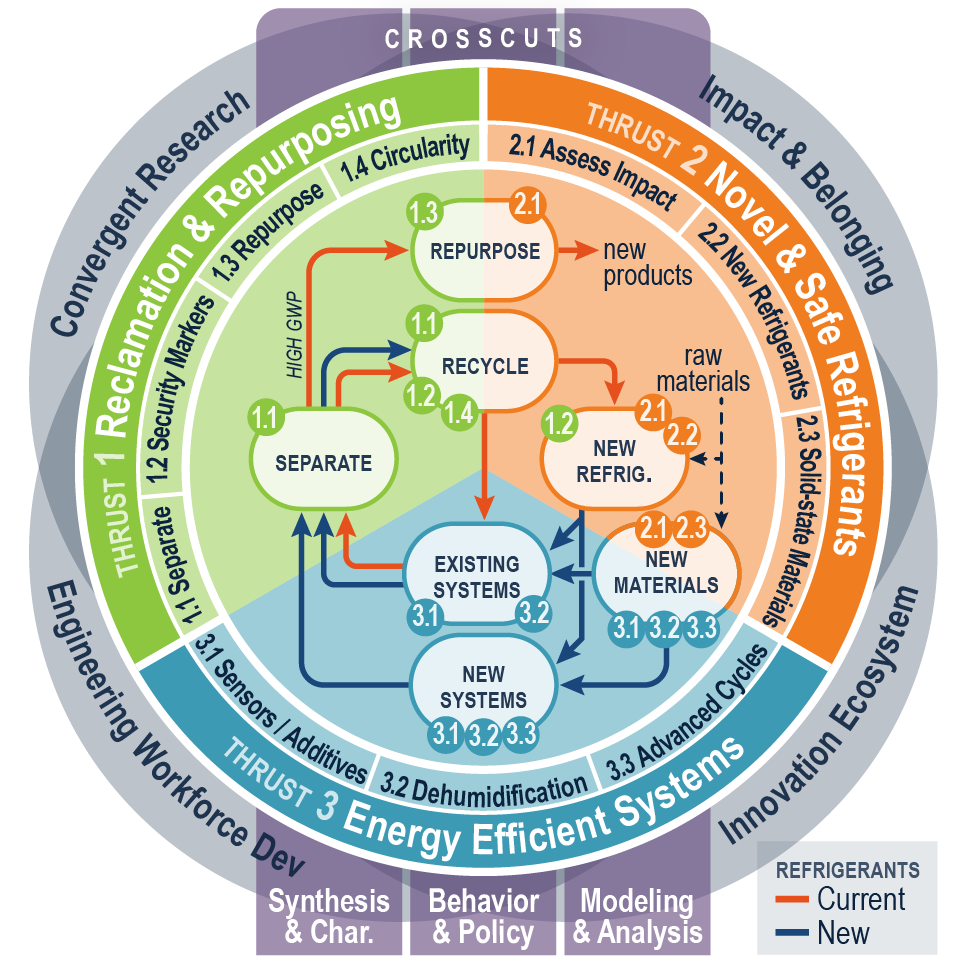
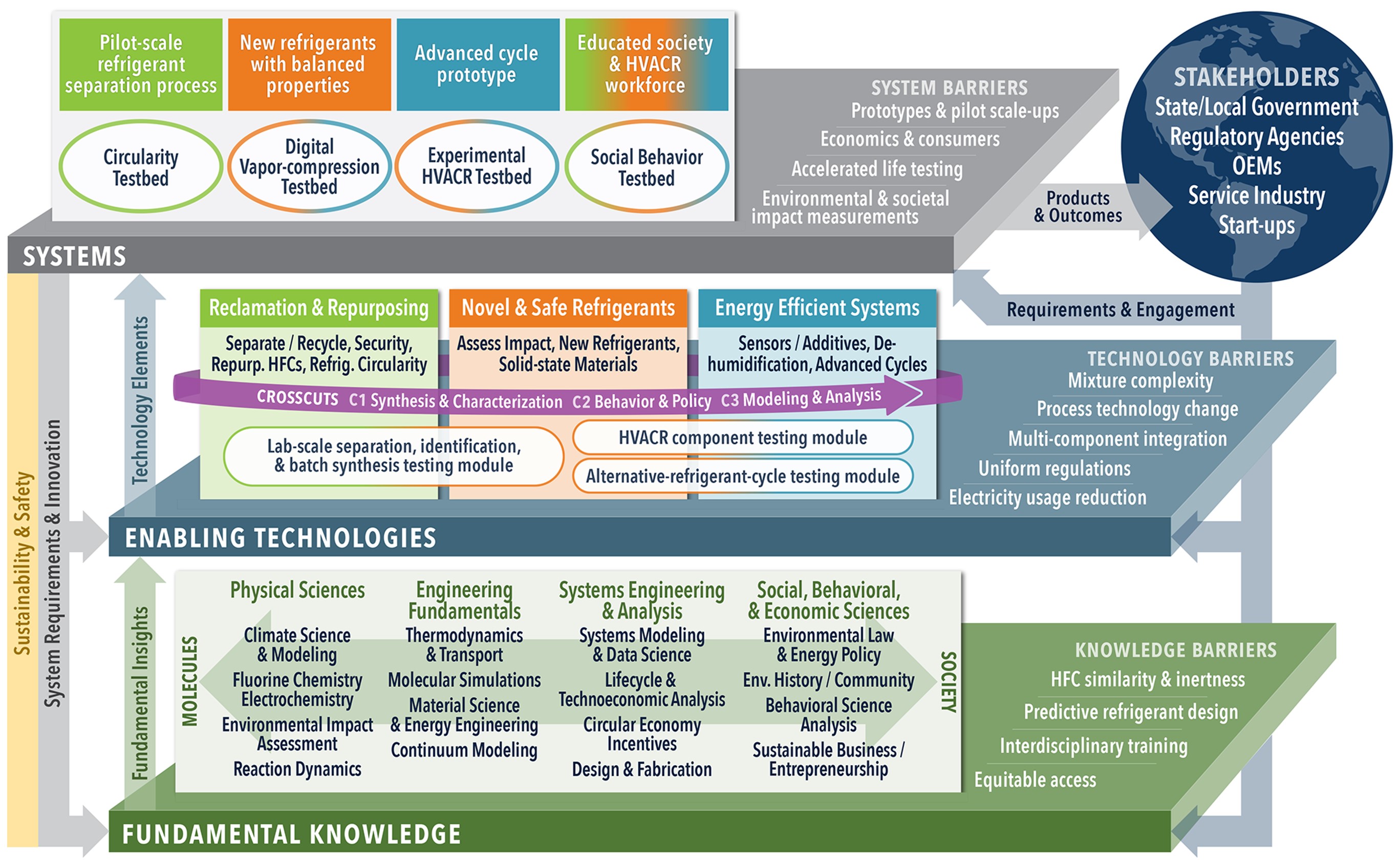
EARTH will confront the scientific, engineering, social, economic, and political issues that are critical to addressing the continually evolving challenges facing the refrigeration and air conditioning ecosystem
1. Develop “transformative refrigerants” that have a balance of properties such as environmental, toxicity, flammability, stability, energy efficiency, system complexity, price, recyclability, and long-term availability
2. Promote the recycling of refrigerants (e.g. billions of kilograms are in use today) so that blend components can be recovered as pure compounds and reused in new more environmentally friendly formulations or repurposed into new materials that are environmentally safe and uniquely functional
3. Develop next-generation cooling technologies with higher energy efficiency than current vapor-compression technologies using high fidelity experiments, advanced atomistic simulations, data science methods, and rigorous process design
EARTH will create the first sustainable refrigerant lifecycle engineered system that tackles the technical, environmental, and societal challenges of the Heating, Ventilation, Air-conditioning, and Refrigeration (HVACR) industry that aligns with national research ideas and the importance of chemical research to the U.S. economy.
technical, environmental, and societal challenges of the Heating, Ventilation, Air-conditioning,
and Refrigeration (HVACR) industry that aligns with national research ideas and the
importance of chemical research to the U.S. economy.
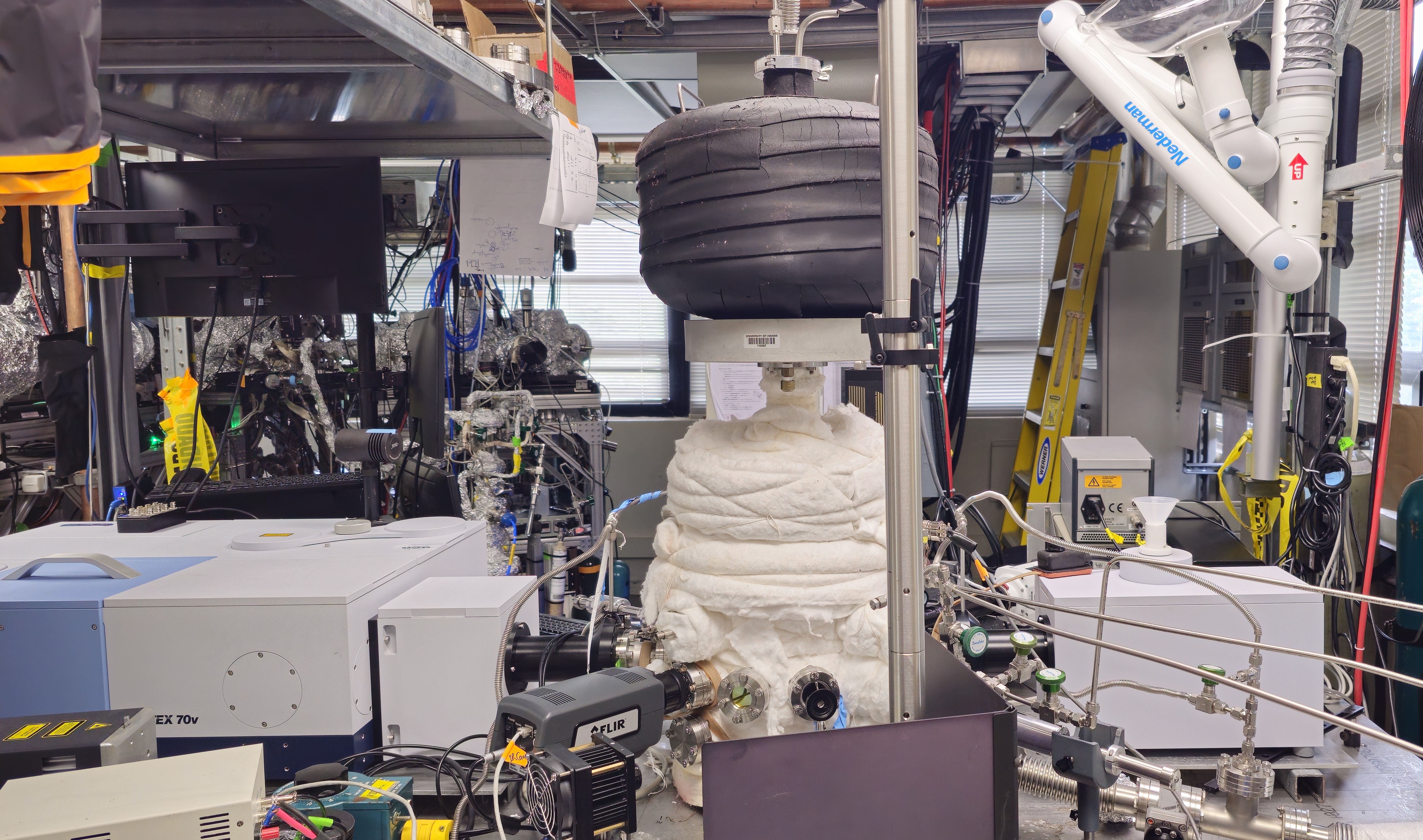
The universal low temperature atmospheric simulation chamber incorporates an ultrasonic levitator surrounded by a cryogenic cooling jacket inside the process chamber. The achieved subzero temperature environment allows experimental investigation of (doped) aqueous droplet freezing to ice crystal in real time to unravel the freezing dynamics through evolution of distinctive molecular structure even in presence of trace gases. This freezing event is ubiquitous in nature and plays a central role in fundamental atmospheric processes such as cloud formation and precipitation including snow and hail via a metastable supercooled state followed by ice nucleation, differing from that of bulk water freezing or freezing events in contact with macroscopic surfaces. Unexplored chemical effects of fluorinated hydrocarbons (HFCs) and hydrofluoroolefins (HFOs) during the aqueous droplet freezing process become vital as dissolution and decomposition both can occur generating harmful poly- and perfluoroalkyl substances (PFASs). Simultaneously, the altered ice nucleation process can cause persistent consequences on the chemistry of refrigerants. Trifluoroacetic acid (TFA) is one of the most common emerging pollutants while its solvation and freezing chemistry in aqueous droplet can answer unsolved question regarding the abnormal presence in Arctic and its transport behavior through the condensed aqueous/ice media. While acoustically levitating, liquid droplets or ice crystals possess the advantage of contactless experimental condition to study the physical and chemical changes probed by spectroscopic (Raman, FTIR, UV-VIS) and camera (optical, thermal) tools surrounding the simulation chamber. Vibrational spectroscopy unfolds the comprehensive molecular interactions of these fluorinated compounds with tetrahedral water or/and hexagonal ice crystalline structural motifs.
The chemistry of the refrigerants on ice surfaces and within ices is not well known. After the Montreal protocol, the ozone depleting cholorofluoro carbons (CFC) were replaced by more environment friendly hydrofluoro carbons (HFC). But the understanding of the refrigerant chemistry in upper troposphere and of their degradation products is still in its infancy. New-generation HFCs, mainly hydrofluoroolefins (HFOs) like 2,3,3,3-tetrafluoropropene are widely utilized in household and automotive industries for cooling systems. However, there is a significant knowledge gap with respect to the fate of these new refrigerants under tropospheric conditions with concerns that they may form persistent chemicals like trifluoroacetic acid. Once released into the troposphere, it is speculated that these refrigerants are degraded eventually to trifluoroacetyl fluoride (CF3CFO), trifluoro acetaldehyde (CF3CHO), and ultimately trifluoro acetic acid (CF3COOH). In the presence of water crystals and in particular on water surfaces, these degradation processes are unknown. These processes are simulated and explored under matrix isolation conditions and on surfaces of water ices in an ultrahigh vacuum vessel.
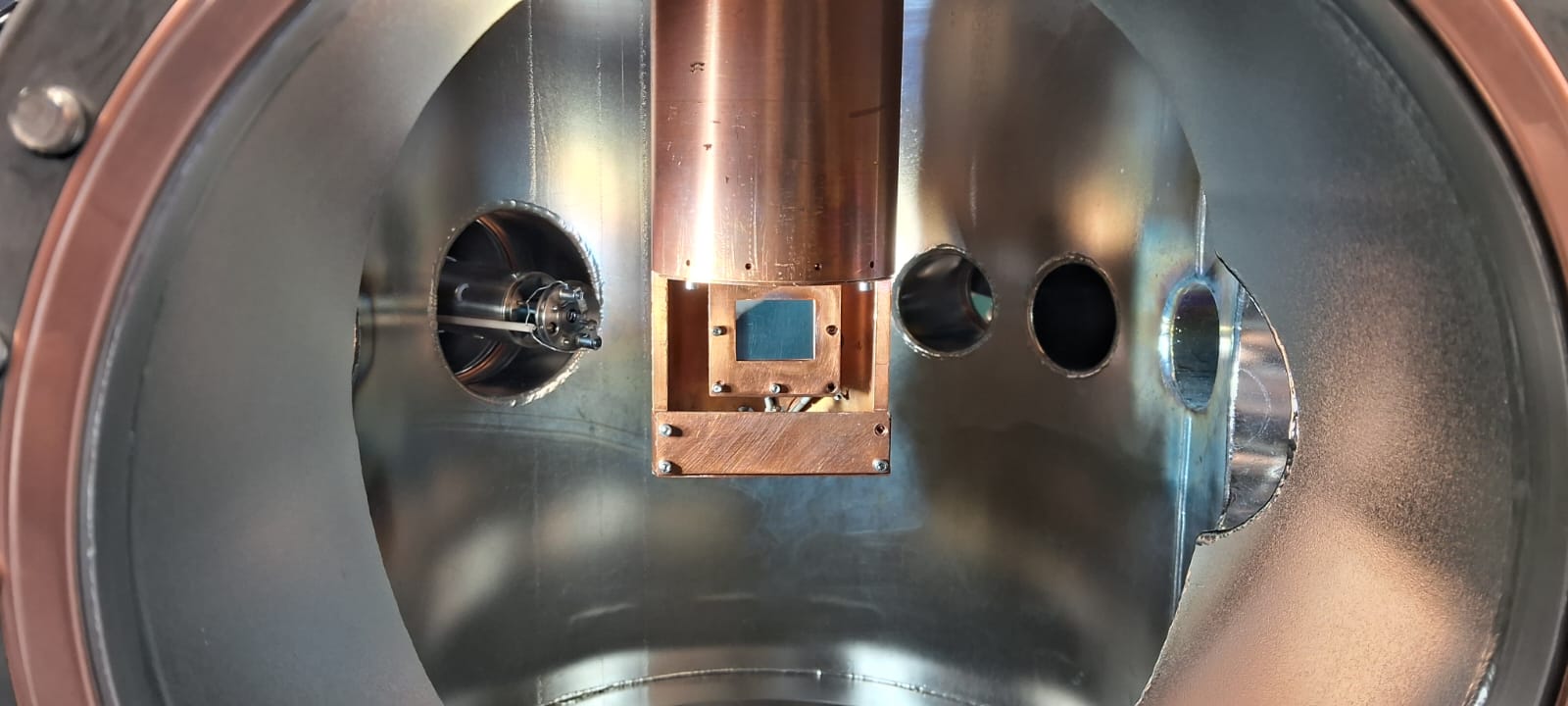
Hydroxyl (OH) radicals play a central role in the atmospheric chemistry of the degradation of hydrofluorocarbons (HFCs). Due to their high reactivity, they initiate a complex chain of gas phase reaction, and hence, there is a concern on how the can affect the chemistry of the troposphere. On the one hand, HFCs have been exploited as refrigerants in air conditioning and refrigeration systems, but key refrigerants represent potent greenhouse gases. On the other hand, new refrigerants are being developed as a more environmentally friendly alternative to HFCs, but laboratory data are needed to investigate the effect of these refrigerants on the atmospheric chemistry. Therefore, it is essential to study in the laboratory the intermediates and products of reactions of refrigerants with hydroxyl radicals, since they are unknown and can lead to the formation of environmentally harmful species. The crossed molecular beams apparatus offers a highly detailed view of the reaction dynamics of these bimolecular processes, allowing to elucidate their entrance and exit barriers, and reaction intermediates, critical for understanding the atmospheric fate of the investigated refrigerants, such as 1,1,1,2-tetrafluoroethane (CF3CH2F) or trifluoropropene (C3F3H3). In these experiments, a primary supersonic beam of hydroxyl radicals interacts with a second supersonic beam containing the refrigerant under single collision conditions. The reactively scattered products are monitored by a triply differentially pumped mass spectrometric detector, rotatable in the scattering plane defined by the primary and secondary beams. At the entrance of the detector the neutral products are ionized by electron impact ionization and filtered according to their mass to charge ratio by a quadrupole mass spectrometer.
Recent Selected Publications
1. S. Biswas, D. Paul, K. Mondal, R. I. Kaiser, Simulating atmospheric freezing of single aqueous droplets to ice in a cryogenically cooled ultrasonic levitator, PNAS, 122, e2425543122 (2025). (PDF) (Supplemental Information) (Data Files)
2. K. Mondal, S. Biswas, N. Melbourne, R. Sun, R. I. Kaiser, Probing the freezing chemistry of singly levitated aqueous trifluoroacetic acid droplets in a cryogenically cooled simulation chamber relevant to Earth's upper troposphere, Chem. Sci., 16, 11039−11048 (2025). (PDF) (Supplemental Information) (Data Files)


