Exploring the Reaction Dynamics of Elementary Reactions Leading to Polycyclic Aromatic Hydrocarbons (PAHs) and their Hydrogen Deficient Precursors
The primary objectives of this project are to experimentally explore the energetics, dynamics, and potential energy surfaces (PESs) of reactions of key aromatic radicals (ARs) with unsaturated C2 to C4 hydrocarbons leading to prototype polycyclic aromatic hydrocarbons (PAHs) carrying six membered and five membered rings in the gas phase under single collision conditions at the most fundamental, microscopic level. These molecular mass growth processes represent a fundamental unsolved challenge and are of critical importance to the gas phase reaction dynamics community to ultimately untangle the formation of three-dimensional carbonaceous nanostructures and soot particles. PAHs carrying five membered rings such as fluorene and cyclopentanaphthalenes represent essential molecular building blocks of non-planar PAHs like corannulene along with fullerenes. These species require five-membered rings in the carbon backbone of the PAH to ‘bent’ PAHs like corannulene out of the plane. The intimate knowledge of the elementary mechanisms to synthesize PAHs carrying five-membered ring(s) is therefore critical to our understanding of the early stage chemistry in combustion systems how precursor PAHs to three dimensional (bowl-shaped) carbonaceous structures and ultimately soot particles form. However, the inherent elementary steps, reaction dynamics, energy flow processes, and reaction mechanisms to form these PAHs on the molecular level are still elusive as detailed synthetic routes have not been investigated experimentally under single collision conditions to date.
To achieve these goals, we conduct two sets of complementary experiments. First, reactions are initiated in a crossed molecular beams machine under single collision conditions by intersecting two supersonic reactant beams containing radicals and closed shell species under a well-defined collision energy and crossing angle (UH Manoa). By recording angular-resolved time of flight (TOF) spectra, we obtain information on the reaction products, intermediates involved, branching ratios of competing reaction channels, reaction energetics, and on the underlying reaction mechanisms to form PAHs along with their acyclic isomers. Second, in collaboration with Dr. Musahid Ahmed (Advanced Light Source, Lawrence Berkeley National Laboratory), reactions have been carried out in a chemical reactor at well characterized pressure and temperature distributions with reaction products interrogated isomer-selectively by tunable vacuum ultraviolet light (VUV) via photoionization (PI) coupled with a reflectron time of flight mass spectrometer (ReTOFMS). Both sets of experiments are merged with electronic structure calculations by Prof. Alexander M. Mebel (Florida International University), and are supported by synthetic efforts Prof. Felix Fischer (UC Berkeley); Prof. Stanislaw Wnuk (Florida International University). This endeavor helps to extract the underlying chemical dynamics, reaction mechanisms, products and intermediates, energetics, branching ratios, and enthalpies of formation of complex polycyclic aromatic hydrocarbons (PAHs). This also assists to comment on the vital role of these processes to form distinct PAHs and their isomers in combustion flames and links to fundamental molecular mass growth processes leading ultimately to soot particles. These data are very much required by the combustion chemistry community to understand the formation of polycyclic aromatic hydrocarbons (PAHs) and their hydrogen deficient precursors from the ‘bottom up’.
Bicyclic Aromatic Hydrocarbons
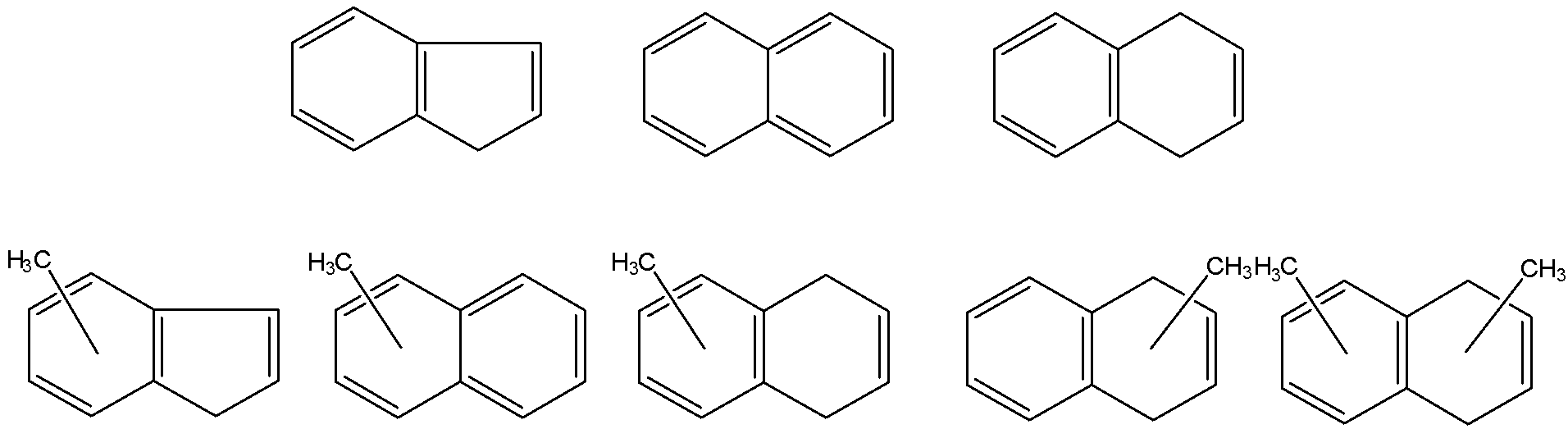
Considering the unique capability of our setups to untangle the energetics and dynamics of bimolecular reactions leading to prototype bicyclic polycyclic aromatic hydrocarbons (PAHs) indene (C9H8), naphthalene (C10H8), and dihydronaphthalene (C10H10) via reactions of the phenyl radical (C6H5) with unsaturated C3 (methylacetylene, allene) and C4 hydrocarbons (vinylacetylene, 1,3-butadiene) under single collision conditions, we expanded our studies to the next level and investigated the formation of (di)methyl-substituted PAHs with indene and naphthalene cores in crossed molecular beams experiments and by exploiting the chemical reactor. In the crossed beams machine, we prepared intense supersonic beams of para- and meta-tolyl (4- and 3-methylphenyl) radicals (C6H4CH3) via photodissociation of helium-seeded para- and meta-chlorotoluene (4- and 3-chlorotoluene) at 193 nm and probed the reactions with unsaturated C3 to C5 hydrocarbons. With the exception of methyl-substituted indene, these bimolecular reactions lead to the formation of (di)methyl-substituted polycyclic aromatic hydrocarbons (PA-Hs) with naphthalene and 1,4-dihydronaphthalene cores in exoergic and entrance barrier-less reactions under single collision conditions. Most importantly, the reaction mechanism involves the initial formation of a van-der-Waals complex and addition of the phenyl-type radical to the C1 position of a vinyl-type group through a submerged barrier. Our investigations suggest that in the hydrocarbon reactant, the vinyl-type group must be in conjugation with a -C≡CH or -HC=CH2 group to form a resonantly stabilized free radical (RSFR) intermediate, which eventually isomerizes to a cyclic intermediate followed by hydrogen loss and aromatization with PAH formation. The barrierless formation of (dimethyl-substituted) PAHs defies conventional wisdom that PAH synthesis necessitates elevated temperatures. Studies in the chemical reactor revealed the complimentary nature of the hydrogen abstraction – acetylene addition (HACA) mechanism leading to naphthalene (C10H8) at elevated temperatures via reactions of phenyl radicals with acetylene; on the other hand, reactions of the phenyl radical with vinylacetylene via the hydrogen abstraction – vinylacetylene addition (HAVA) mechanism synthesize naphthalene (C10H8) without entrance barrier at low temperatures as well. These systems were also expanded to explore the formation of nitrogen-bearing PAH counterparts.
Formation of Tricyclic Aromatic Hydrocarbons
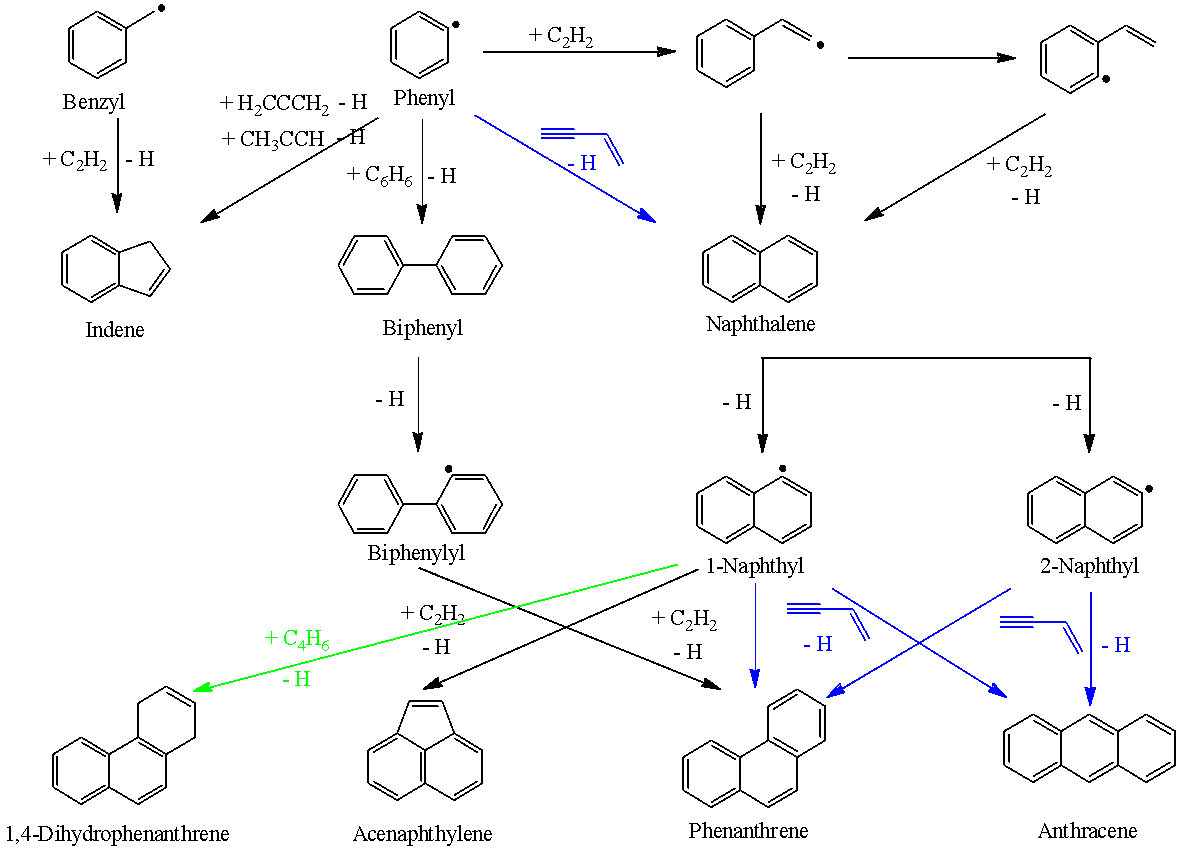
We elucidated fundamental reaction mechanisms leading to the formation of three-ring PAHs carrying three six membered rings [phenanthrene (C14H10), dihydrophenanthrene (C14H12), and anthracene (C14H10)] and two six membered plus one five membered ring [acenaphthylene (C12H8)]. These studies revealed that the HACA mechanism neither yields anthracene (C14H10) nor phenanthrene (C14H10) from naphthyl radical reactions (C10H7) with acetylene, but solely acenaphthylene (C12H8) However, HACA starting from non-PAH radicals such as biphenylyl (C12H9) reacting with acetylene (C2H2) can synthesize via bay-closure phenanthrene (C14H10), but not the anthracene isomer (C14H10). On the other hand, HAVA commencing from 1- and 2- naphthyl radicals (C10H7) reacting with vinylacetylene (C4H4) can form via ring annulation phenanthrene (C14H10) and anthracene (C14H10) via barrierless reactions of aryl-type aromatic radicals with vinylacetylene. These studies highlight the complementary nature of HACA and HAVA to form two dimensional (planar) PAHs via bay-closure and barrierless ring annulation with HAVA operating even at ultralow temperatures.
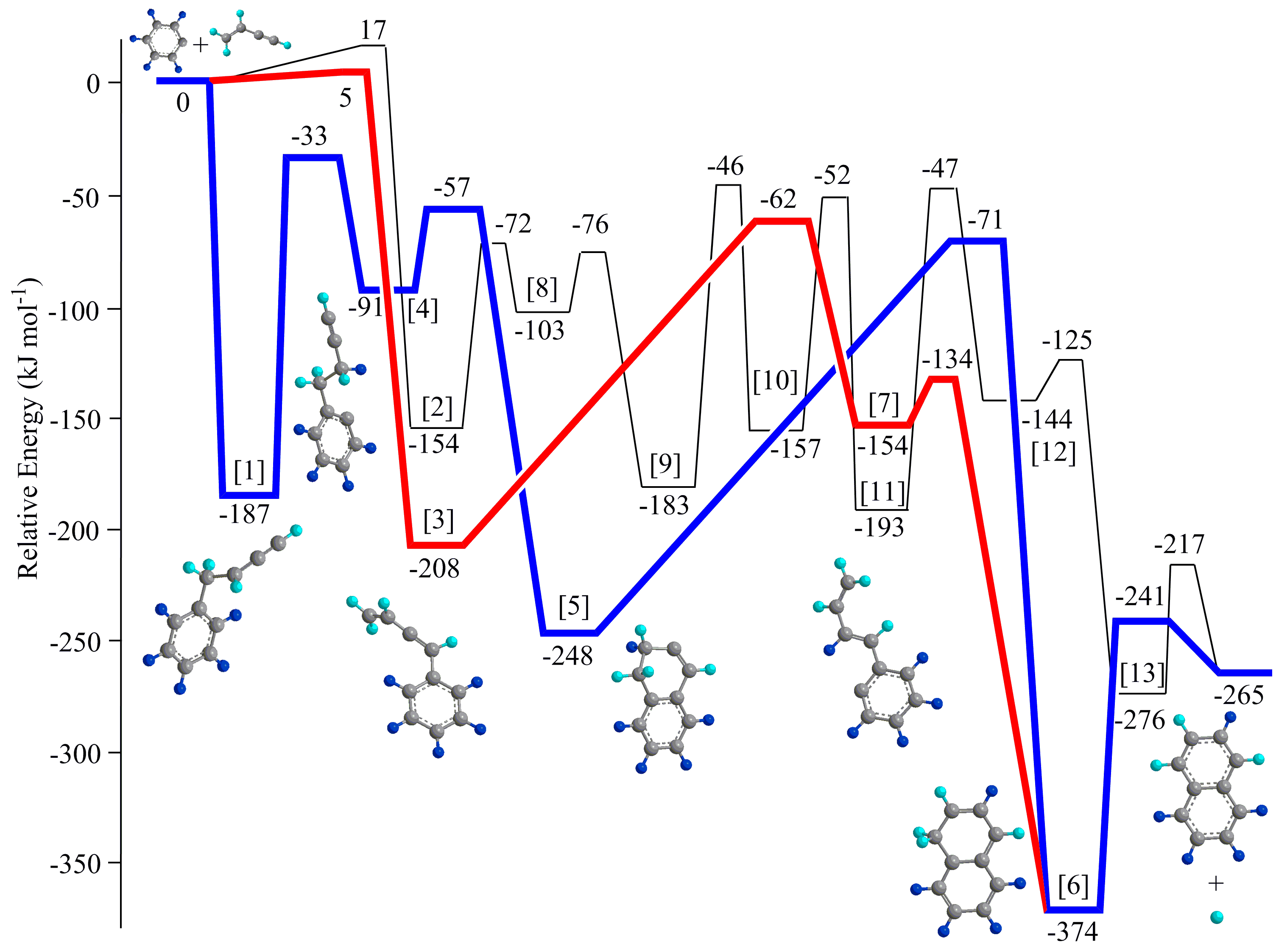
Formation of Tetracyclic Aromatic Hydrocarbons
We expanded our studies on the growth of PAHs from bi- and tricyclic systems to aromatic systems carrying four six membered rings utilizing pyrene (C16H10) as a prototype. By exploring the reaction of the 4-phenanthrenyl radical ([C14H9]) with acetylene (C16H10) under conditions prevalent in high temperature combustion systems, we provide testimony on a facile, isomer-selective formation of pyrene (C16H10). Along with the Hydrogen Abstraction – Vinylacetylene Addition (HAVA) mechanism, molecular mass growth processes from pyrene may lead through systematic ring annulation not only to more complex PAHs, but ultimately to two-dimensional graphene-type nanostructures thus facilitating an understanding toward soot growth in combustion systems.
Recent Selected Publications
1. A. M. Mebel, V.V. Kislov, R.I. Kaiser, Photoinduced Mechanism of Formation and Growth of Polycyclic Aromatic Hydrocarbons in Low-Temperature Environments via Successive Ethynyl Radical Additions, JACS 130, 13618-13629 (2008). (PDF)
2. F. Zhang, B. Jones, P. Maksyutenko, R.I. Kaiser, C. Chin, V.V. Kislov, A.M. Mebel, Formation of the Phenyl Radical [C6H5(X2A1)] under Single Collision Conditions: A Crossed Molecular Beam and Ab Initio Study, JACS 132, 2672-2683 (2010). (PDF)
3. B.M. Jones, F. Zhang, R.I. Kaiser, A. Jamal, A.M. Mebel, M.A. Cordiner, S.B. Charnley, Formation of Benzene in the Interstellar Medium, Proceedings of the National Acadamy of Sciences USA 108, 452-457 (2011). (PDF)
4. D.S.N. Parker, F. Zhang, Y.S. Kim, R.I. Kaiser, A. Landera, V.V. Kislov, A.M. Mebel, A.G.G.M. Tielens, Low Temperature Formation of Naphthalene and its Role in the Synthesis of PAHs (Polycyclic Aromatic Hydrocarbons) in the Interstellar Medium, PNAS, 109, 53-58 (2012). (PDF)
5. B.B. Dangi, D.S.N. Parker, R.I. Kaiser, A. Jamal, A.M. Mebel, A Combined Experimental and Theoretical Study on the Gas-Phase Synthesis of Toluene under Single Collision Conditions, Angew. Chem. Int. Ed., 52, 7186-7189 (2013). (PDF)
6. B.B. Dangi, D.S.N. Parker, T. Yang, R.I. Kaiser, A.M. Mebel, Gas Phase Synthesis of the Benzyl Radical (C6H5CH2), Angew. Chem. Int. Ed., 53, 4608-4613 (2014). (PDF)
7. D.S.N. Parker, R.I. Kaiser, T.P. Troy, M. Ahmed, Hydrogen Abstraction/Acetylene Addition Revealed, Angew. Chem. Int. Ed., 53, 7740-7744 (2014). (PDF)
8. R.I. Kaiser, D. S. N. Parker, A. M. Mebel, Reaction Dynamics in Astrochemistry: Low-Temperature Pathways to Polycyclic Aromatic Hydrocarbons in the Interstellar Medium, Annual Reviews of Physical Chemistry. 66, 43-47 (2015). (PDF)
9. T. Yang, L. Muzangwa, D.S.N. Parker, R.I. Kaiser, A.M. Mebel, Formation of 2- and 1-Methyl-1,4-Dihydronaphthalene Isomers via the Crossed Beam Reactions of Phenyl Radicals (C6H5) with Isoprene (CH2C(CH3)CHCH2) and 1,3-Pentadiene (CH2CHCHCHCH3), Phys. Chem. Chem. Phys., 17, 530-540 (2015). (PDF)
10. L. Muzangwa, T. Yang, D.S.N. Parker, R.I. Kaiser, A.M. Mebel, A. Jamal, M. Ryazantsev, K. Morokuma, A Crossed Molecular Beam and Ab Initio Study on the Formation of 5- and 6-Methyl-1,4-Dihydronaphthalene (C11H12) via the Reaction of Meta-Tolyl (C7H7) with 1,3-Butadiene (C4H6), Phys. Chem. Chem. Phys., 17, 7699-7706 (2015). (PDF)
11. T. Yang, D.S.N. Parker, B.B. Dangi, R.I. Kaiser and A.M. Mebel, Formation of 5- and 6-methyl-1H-indene (C10H10) via the Reactions of the Para-tolyl Radical (C6H4CH3) with Allene (H2CCCH2) and Methylacetylene (HCCCH3) under Single Collision Conditions, Phys. Chem. Chem. Phys., 17, 10510-10519 (2015). (PDF)
12. D.S.N. Parker, R.I. Kaiser, O. Kostko, T.P. Troy, M. Ahmed, A.M. Mebel, A.G.G.M. Tielens, Gas Phase Synthesis of (Iso)Quinoline and Its Role in the Formation of Nucleobases in the Interstellar Medium, Ap J., 803:53, 1-10 (2015). (PDF)
13. D.S.N. Parker, R.I. Kaiser, B. Bandyopadhyay, O. Kostko, T.P. Troy, M. Ahmed, Unexpected Chemistry from the Reaction of Naphthyl and Acetylene at Combustion-Like Temperatures, Angew. Chem. Int. Ed. 54, 5421-5424 (2015). (PDF)
14. D.S.N. Parker, R.I. Kaiser, O. Kostko and M. Ahmed, Selective Formation of Indene through the Reaction of Benzyl Radicals with Acetylene, Chem. Phys. Chem. 16, 2091-2093 (2015). (PDF)
15. D.S.N. Parker, R.I. Kaiser, T.P. Troy, O. Kostko and M. Ahmed, Toward the Oxidation of the Phenyl Radical and Prevention of PAH Formation in Combustion Systems, J. Phys. Chem. A 119, 7145-7154 (2015). (PDF)
16. T. Yang, L. Muzangwa, R.I. Kaiser, A. Jamal, and K. Morokuma, A Combined Crossed Molecular Beam and Theoretical Investigation of the Reaction of the Meta-tolyl Radical with Vinylacetylene- toward the Formation of Methylnaphthalenes, Phys. Chem. Chem. Phys. 34, 461-514 (2015). (PDF)
17. A.M. Mebel, R.I. Kaiser, Formation of resonantly stabilised free radicals via the reactions of atomic carbon, dicarbon, and tricarbon with unsaturated hydrocarbons: theory and crossed molecular beams experiments, International Reviews in Physical Chemistry. 17, 21564-21575 (2015). (PDF)
18. D.S.N. Parker, R.I. Kaiser, O. Kostko, T.P. Troy, M. Ahmed, B-J Sun, S-H Chen, A.H.H. Chang, On the formation of pyridine in the interstellar medium, Phys. Chem. Chem. Phys., 17, 32000-32008 (2015). (PDF)
19. D.S.N. Parker, T. Yang, B.B. Dangi, R.I. Kaiser, P.P. Bera, and T.J. Lee, Low Temperature Formation of Nitrogen-substituted Polycyclic Aromatic Hydrocarbons (PANHs)- Barrierless Routes to Dihydro(iso)quinolines, Ap. J., 815, 11 (2015). (PDF)
20. T. Yang, T.P. Troy, B. Xu, O. Kostko, M. Ahmed, A.M. Mebel, R.I. Kaiser, Hydrogen-Abstraction/Acetylene-Addition Exposed, Angew. Chem. Int. Ed., 55, 14983-14987 (2016). (PDF)
21. A.M. Thomas, T. Yang, B.B. Dangi, R.I. Kaiser, G-S Kim, A.M. Mebel Oxidation of the para-Tolyl Radical by Molecular Oxygen under Single-Collison Conditions: Formation of the para-Toloxy Radical, J. Phys. Chem. Lett., 7, 5121-5127 (2016). (PDF)
22. D.S.N. Parker, R.I. Kaiser, On the Formation of Nitrogen-Substituted Polycyclic Aromatic Hydrocarbons (NPAHs) in Circumstellar and Interstellar Environments, Chem. Soc. Rev., 46, 452-463 (2017). (PDF)
23. A.M. Mebel, A. Landera, R.I. Kaiser, Formation Mechanisms of Naphthalene and Indene: From the Interstellar Medium to Combustion Flames, J. Phys. Chem. A, 121, 901-926 (2017). (PDF)
24. T. Yang, R.I. Kaiser, T.P. Troy, B. Xu, O. Kostko, M. Ahmed, A.M. Mebel, M.V. Zagidullin, V.N. Azyazov, HACA's Heritage: A Free-Radical Pathway to Phenanthrene in Circumstellar Envelopes of Asymptotic Giant Branch Stars, Angew. Chem. Int. Ed., 56, 4515-4519 (2017). (PDF)
25. A.M. Thomas, M. Lucas, T. Yang, R.I. Kaiser, L. Fuentes, D. Belisario-Lara, A.M. Mebel, A Free-Radical Pathway to Hydrogenated Phenanthrene in Molecular Clouds - Low Temperature Growth of Polycyclic Aromatic Hydrocarbons, Chem. Phys. Chem., 18, 1971-1976 (2017). (PDF)
26. M. Lucas, A.M. Thomas, L. Zhao, R.I. Kaiser, G-S Kim, A.M. Mebel, Gas-Phase Synthesis of the Elusive Cyclooctatetraenyl Radical (C8H7) via Triplet Aromatic Cyclooctatetraene (C8H8) and Non-Aromatic Cyclooctatriene (C8H8) Intermediates, Angew. Chem. Int. Ed., 56, 13655-13660 (2017). (PDF)
27. M. Lucas, A.M. Thomas, R.I. Kaiser, E.K. Bashkirov, V.N. Azyazov, A.M. Mebel Combined Experimental and Computational Investigation of the Elementary Reaction of Ground State Atomic Carbon (C; 3Pj) with Pyridine (C5H5N; X1A1) via Ring Expansion and Ring Degradation Pathways, J. Phys. Chem. A 122, 3128-3139 (2018). (PDF)
28. A.M. Thomas, M. Lucas, L. Zhao, J. Liddiard, R.I. Kaiser, A.M. Mebel A combined crossed molecular beams and computational study on the formation of distinct resonantly stabilized C5H3 radicals via chemically activated C5H4 and C6H6 intermediates, Phys. Chem. Chem. Phys. 20, 10906-10925 (2018). (PDF)
29. L. Zhao; R.I. Kaiser, B. Xu, U. Ablikim, M. Ahmed, D. Joshi, G. Veber, F.R. Fischer, A.M. Mebel Pyrene synthesis in circumstellar envelopes and its role in the formation of 2D nanostructures, Nature Astronomy 2, 413-419 (2018). (PDF)
30. L. Zhao, R.I. Kaiser, B. Xu, U. Ablikim, M. Ahmed, M.V. Zagidullin, V.N. Azyazov, A.H. Howlader, S.F. Wnuk, A.M. Mebel VUV Photoionization Study on the Formation of the Simplest Polycyclic Aromatic Hydrocarbon: Naphthalene (C10H8), J. Phys. Chem. Lett. 9, 2620-2626 (2018). (PDF)
31. A.M. Thomas, L. Zhao, C. He, A.M. Mebel, R.I. Kaiser, A Combined Experimental and Computational Study on the Reaction Dynamics of the 1-Propynyl (C3H3)-Acetylene (HCCH) System and the Formation of Methyldiacetylene (CH3CCCCH), J. Phys. Chem. A 122, 6663-6672 (2018). (PDF)
32. L. Zhao, R.I. Kaiser, B. Xu, U. Ablikim, M. Ahmed, M.M. Evseev, E.K. Bashkirov, V.N. Azyazov, A.M. Mebel, Low-Temperature Formation of Polycyclic Aromatic Hydrocarbons in Titan's Atmosphere, Nat. Astron. 2, 973–979 (2018). (PDF)
33. M.V. Zagidullin, R.I. Kaiser, D.P. Porfiriev, I.P. Zavershinskiy, M. Ahmed, V.N. Azyazov, A.M. Mebel, Functional Relationships between Kinetic, Flow, and Geometrical Parameters in a High-Temperature Chemical Microreactor, J. Phys. Chem. A, 8819–8827 (2018). (PDF)
34. L. Zhao, R.I. Kaiser, B. Xu, U. Ablikim, W. Lu, M. Ahmed, M.M. Evseev, E.K. Bashkirov, V.N. Azyazov, A.H. Howlader, S.F. Wnuk, A.M. Mebel, Gas-Phase Synthesis of Triphenylene (C18H12), ChemPhysChem 20, 791-797 (2019). (PDF)
35. L. Zhao, R.I. Kaiser, B. Xu, U. Ablikim, W. Lu, M. Ahmed, M.M. Evseev, E.K. Bashkirov, V.N. Azyazov, M.V. Zagidullin, A.N. Morozov, A.H. Howlader, S.F. Wnuk, A.M. Mebel, D. Joshi, G. Veber, F.R. Fischer, Gas phase synthesis of [4]-helicene, Nat. Commun. 10, 1510-1517 (2019). (PDF)
36. A. M. Thomas, C. He, L. Zhao, G. R. Galimova, A. M. Mebel, R. I. Kaiser, Combined Experimental and Computational Study on the Reaction Dynamics of the 1-Propynyl (CH3CC)-1,3-Butadiene (CH2CHCHCH2) System and the Formation of Toluene under Single Collision Conditions, J. Phys. Chem. A, 123, 4104-4118, (2019). (PDF)
37. L. Zhao, M.B. Prendergast, R.I. Kaiser, B. Xu, W. Lu, U. Ablikim, M. Ahmed, A.D. Oleinikov, V.N. Azyazov, A.M. Mebel, A.H. Howlader, S.F. Wnuk, Reactivity of the Indenyl Radical (C9H7) with Acetylene (C2H2) and Vinylacetylene (C4H4), ChemPhysChem, 20, 1437-1447 (2019). (PDF)
38. C. He, L. Zhao, A. M. Thomas, A. N. Morozov, A. M. Mebel, R. I. Kaiser, Elucidating the Chemical Dynamics of the Elementary Reactions of the 1-Propynyl Radical (CH3CC; X2A1) with Methylacetylene (H3CCCH; X1A1) and Allene (H2CCCH2; X1A1), J. Phys. Chem. A, 123, 5446-5462 (2019). (PDF)
39. L. Zhao, M. Prendergast, R.I. Kaiser, B. Xu, U. Ablikim, W. Lu, M. Ahmed, A.D. Oleinikov, V.N. Azyazov, A.H. Howlader, S.F. Wnuk, A.M. Mebel, How to add a five-membered ring to polycyclic aromatic hydrocarbons (PAHs) – molecular mass growth of the 2-naphthyl radical (C10H7) to benzindenes (C13H10) as a case study, Phys. Chem. Chem. Phys. 21, 16737-16750 (2019). (PDF)
40. C. He, A. M. Thomas, G. R. Galimova, A.M. Mebel, R.I. Kaiser, Gas Phase Formation of the Interstellar Molecule Methyltriacetylene, ChemPhysChem, 20, 1912-1917 (2019). (PDF)
41. L. Zhao, R.I. Kaiser, W. Lu, B. Xu, M. Ahmed, A.N. Morozov, A.M. Mebel, A.H. Howlader, S.F. Wnuk, Molecular mass growth through ring expansion in polycyclic aromatic hydrocarbons via radical–radical reactions, Nat. Commun. 10, 3689 (2019). (PDF)
42. A. M. Thomas, L. Zhao, C. He, G. R. Galimova, A. M. Mebel, R. I. Kaiser, Directed Gas-Phase Synthesis of Triafulvene under Single-Collision Conditions, Angew. Chem., Int. Ed., 58, 15488-15495 (2019). (PDF)
43. C. He, L. Zhao, A. M. Thomas, G. R. Galimova, A. M. Mebel, R. I. Kaiser, A Combined Experimental and Computational Study on the Reaction Dynamics of the 1-Propynyl Radical (CH3CC; X2A1) with Ethylene (H2CCH2; X1A1g) and the Formation of 1-Penten-3-yne (CH2CHCCCH3; X1A'), Phys. Chem. Chem. Phys, 21, 22308-22319 (2019). (PDF)
44. L. Zhao, M. B. Prendergast, R. I. Kaiser, B. Xu, U. Ablikim, M. Ahmed, B-J Sun, Y-L Chen, A. H. H. Chang, R. K. Mohamed, F. R. Fischer, Synthesis of Polycyclic Aromatic Hydrocarbons by Phenyl Addition–Dehydrocyclization: The Third Way, Angew. Chem. Int. Ed. 58, 17442-17450 (2019). (PDF)
45. A. M. Thomas, S. Doddipatla, R. I. Kaiser, G. R. Galimova, A. M. Mebel A Barrierless Pathway Accessing the C9H9 and C9H8 Potential Energy Surfaces via the Elementary Reaction of Benzene with 1-Propynyl, Sci. Rep. 9, 17595 (2019). (PDF)
46. C. He, A. M. Thomas, G. R. Galimova, A. M. Mebel, R. I. Kaiser, Gas-Phase Formation of 1-Methylcyclopropene and 3-Methylcyclopropene via the Reaction of the Methylidyne Radical (CH; X2Π) with Propylene (CH3CHCH2; X1A'), J. Phys. Chem. A 123, 10543-10555 (2019). (PDF)
47. C. He, A. M. Thomas, G. R. Galimova, A. N. Morozov, A. M. Mebel, R. I. Kaiser, Gas-Phase Formation of Fulvenallene (C7H6) via the Jahn-Teller Distorted Tropyl (C7H7) Radical Intermediate under Single-Collision Conditions, JACS 142, 3205-3213 (2020). (PDF)
48. L. Zhao, R. I. Kaiser, B. Xu, U. Ablikim, M. Ahmed, M. M. Evseev, E. K. Bashkirov, V. N. Azyazov, A. M. Mebel, A Unified Mechanism on the Formation of Acenes, Helicenes, and Phenacenes in the Gas Phase, Angew. Chem. Int. Ed., 59, 4051-4058 (2020). (PDF)
49. C. He, L. Zhao, S. Doddipatla, A. M. Thomas, A. A. Nikolayev, G. R. Galimova, V. N. Azyazov, A. M. Mebel, R. I. Kaiser, Gas-Phase Synthesis of 3-Vinylcyclopropene via the Crossed Beam Reaction of the Methylidyne Radical (CH; X2Π) with 1,3-Butadiene (CH2CHCHCH2; X1Ag), ChemPhysChem, 21, 1295-1309 (2020). (PDF)
50. L. Zhao, R. I. Kaiser, W. Lu, M. Ahmed, M. M. Evseev, E. K. Bashkirov, V. N. Azyazov, C. Tönshoff, F. Reicherter, H. F. Bettinger, A. M. Mebel, A Free Radical Prompted Barrierless Gas-Phase Synthesis of Pentacene, Angew. Chem. Int. Ed. 59, 11334–11338 (2020). (PDF)
51. L. Zhao, R. I. Kaiser, W. Lu, M. Ahmed, A. D. Oleinikov, V. N. Azyazov, A. M. Mebel, A. H. Howlader, S. F. Wnuk, Gas phase formation of phenalene via 10π-aromatic, resonantly stabilized free radical intermediates, Phys. Chem. Chem. Phys. 22, 15381-15388 (2020). (PDF)
52. L. Zhao, R. I. Kaiser, W. Lu, O. Kostko, M. Ahmed, M. M. Evseev, E. K. Bashikirov, A. D. Oleinikov, V. N. Azyazov, A. M. Mebel, A. H. Howlader, S. F. Wnuk, Gas phase formation of cyclopentanaphthalene (benzindene) isomers via reactions of 5- and 6-indenyl radicals with vinylacetylene, Phys. Chem. Chem. Phys. 22, 22493-22500 (2020). (PDF)
53. A. H. Howlader, K. Diaz, A. M. Mebel, R. I. Kaiser, S. F. Wnuk Iodoindenes: Synthesis and application to cross-coupling, Tetrahedron Lett., 61, 152427 (2020). (PDF)
54. C. He, G. R. Galimova, Y. Luo, L. Zhao, A. K. Eckhardt, R. Sun, A. M. Mebel, R. I. Kaiser, A Chemical Dynamics Study on the Gas-phase Formation of Triplet and Singlet C5H2 Carbenes, Proc. Natl. Acad. Sci. U.S.A. 117, 30142-30150 (2020). (PDF)
55. S. Doddipatla, G. R. Galimova, H. Wei, A. M. Thomas, C. He, Z. Yang, A. N. Morozov, C. N. Shingledecker, A. M. Mebel, R. I. Kaiser, Low-temperature Gas-phase Formation of Indene in the Interstellar Medium, Sci. Adv., 7, eabd4044 (2021). (PDF)
56. C. He, A. A. Nikolayev, L. Zhao, A. M. Thomas, S. Doddipatla, G. R. Galimova, V. N. Azyazov, A. M. Mebel, R. I. Kaiser, Gas-Phase Formation of C5H6 Isomers via the Crossed Molecular Beam Reaction of the Methylidyne Radical (CH; X2Π) with 1,2-Butadiene (CH3CHCCH2; X1A'), J. Phys. Chem. A 125, 126-138 (2021). (PDF)
57. L. Zhao, S. Doddipatla, R. I. Kaiser, W. Lu, O. Kostko, M. Ahmed, L. B. Tuli, A. N. Morozov, A. H. Howlader, S. F. Wnuk, A. M. Mebel, V. N. Azyazov, R. K. Mohamed, F. R. Fischer, Gas-phase synthesis of corannulene - a molecular building block of fullerenes, Phys. Chem. Chem. Phys., 23, 5740-5749 (2021). (PDF)
58. R. I. Kaiser, N. Hansen, An Aromatic Universe−A Physical Chemistry Perspective, J. Phys. Chem. A., (2021). doi: 10.1021/acs.jpca.1c00606 (PDF)
59. L. Zhao, W. Lu, M. Ahmed, M. V. Zagidullin, V. N. Azyazov, A. N. Morozov, A. M. Mebel, R. I. Kaiser, Gas-phase synthesis of benzene via the propargyl radical self-reaction, Science Advances, 7, eabf0360 (2021). (PDF)
60. L. Zhao, M. Prendergast, R. I. Kaiser, B. Xu, W. Lu, M. Ahmed, A. H. Howlader, S. F. Wnuk, A. S. Korotchenko, M. M. Evseev, E. K. Bashkirov, V. N. Azyazovde, A. M. Mebel, A molecular beam and computational study on the barrierless gas phase formation of (iso)quinoline in low temperature extraterrestrial environments, Phys. Chem. Chem. Phys., 23, 18495 (2021). (PDF)
61. A. A. Nikolayev, V. N. Azyazov, R. I. Kaiser, A. M. Mebel, Theoretical Study of the Reaction of the Methylidyne Radical (CH; X2Π) with 1-Butyne (CH3CH2CCH; X1A’). J. Phys. Chem A 125, 9536-9547 (2021). (PDF)
62. C. He, K. Fujioka, A. A. Nikolayev, L. Zhao, S. Doddipatla, V. N. Azyazov, A. M. Mebel, R. Sun, R. I. Kaiser, A Chemical Dynamics Study of the Reaction of the Methylidyne Radical (CH, X2Π) with Dimethylacetylene (CH3CCCH3, X1A1g), Phys. Chem. Chem. Phys., 24, 578 (2022). (PDF)
63. R. I. Kaiser, L. Zhao, W. Lu, M. Ahmed, M. V. Zagidullin, V.N. Azyazov, A.M. Mebel, Gas-Phase Formation of Benzene (C6H6) and Naphthalene (C10H8) through Resonantly Stabilized Cyclopentadienyl-Mediated Radical-Radical Reactions. J. Phys. Chem Lett. 13, 208-213 (2022). (PDF)
64. R. I. Kaiser, L. Zhao, W. Lu, M. Ahmed, V. S. Krasnoukhov, V. N. Azyazov, A. M. Mebel, Unconventional Excited-State Dynamics in the Concerted Benzyl (C7H7) Radical Self-Reaction to Anthracene (C14H10). Nat. Commun. 13, 786 (2022). (PDF)
65. S. J. Goettl, C. He, D. Paul, A. A. Nikolayev, V. N. Azyazov, A. M. Mebel, R. I. Kaiser, Gas-Phase Study of the Elementary Reaction of the D1-Ethynyl Radical (C2D; X2Σ+) with Propylene (C3H6; X1A') under Single-Collision Conditions. J. Phys. Chem. A., 126, 1889-1898 (2022). (PDF)
66. D. Paul, Z. Yang, S. J. Goettl, A. M. Thomas, C. He, A. G. Suits, D. H. Parker, R. I. Kaiser, Photodissociation Dynamics of Astrophysically Relevent Propyl Derivatives (C3H7X; X = CN, OH, HCO) at 157 nm Exploiting an Ultracompact Velocity Map Imaging Spectrometer: The (Iso)Propyl Channel, J. Phys. Chem. A, 126, 5768-5775 (2022). (PDF)
67. G. Galimova, A. Mebel, S. J. Goettl, Z. Yang, R. I. Kaiser, A Crossed Molecular Beams and Computational Study on the Unusual Reactivity of Banana Bonds of Cyclopropane (c-C3H6; X1A1') Through Insertion by Ground State Carbon Atoms (C(3Pj)), Phys. Chem. Chem. Phys., 24, 22453-22463 (2022). (PDF)
68. D. Paul, Z. Yang, A. G. Suits, D. H. Parker, R. I. Kaiser, Photodissociation Dynamics of Xylene Isomers C6H4(CH3)2 at 157 nm using an Ultracompact Velocity Map Imaging Spectrometer – The C7H7 Channel., Chem. Phys. Lett., 807, 140064 (2022). (PDF)
69. R. I. Kaiser, L. Zhao, W. Lu, M. Ahmed, M. M. Evseev, V. N. Azyazov, A. M. Mebel, R. K. Mohamed, F. R. Fischer, X. Li Gas-phase synthesis of racemic helicenes and their potential role in the enantiomeric enrichment of sugars and amino acids in meteorites, Phys. Chem. Chem. Phys., 24, 25077-25087 (2022). (PDF)
70. C. He, Z. Yang, S. Doddipatla, A. M. Thomas, R. I. Kaiser, G. R. Galimova, A. M. Mebel, K. Fujioka, R. Sun, Directed Gas Phase Preparation of Ethynylallene (H2CCCHCCH; X1A') via the Crossed Molecular Beam Reaction of the Methylidyne Radical (CH; X2Π) with Vinylacetylene (H2CCHCCH; X1A'), Phys. Chem. Chem. Phys., 24, 26499-26510 (2022). (PDF)
71. Z. Yang, G. R. Galimova, C. He, S. Doddipatla, A. M. Mebel, R. I. Kaiser, Gas Phase Formation of 1,3,5,7-Cyclooctatetraene (C8H8) through Ring Expansion via the Aromatic 1,3,5-Cyclooctatrien-7-yl Radical (C8H9•) Transient , JACS, 144, 22470-22478 (2022). (PDF)
72. W. Li, L. Zhao, R. I. Kaiser, A Unified Reaction Network on the Formation of Five-Membered Ringed Polycyclic Aromatic Hydrocarbons (PAHs) and Their Role in Ring Expansion Processes Through Radical-Radical Reactions, Phys. Chem. Chem. Phys., 25, 4141-4150 (2023). (PDF)
73. C. He, R. I. Kaiser, W. Lu, M. Ahmed, P. S. Pivovarov, O. V. Kuznetsov, M. V. Zagidullin, A. M. Mebel, Unconventional Pathway in the Gas-Phase Synthesis of 9H-Fluorene (C13H10) via the Radical-Radical Reaction of Benzyl (C7H7) with Phenyl (C6H5), Angew. Chem. Int. Ed. 62, e202216972 (2023). (PDF)
74. C. He, R. I. Kaiser, W. Lu, M. Ahmed, Y. Reyes, S. F. Wnuk, A. M. Mebel, Exotic Reaction Dynamics in the Gas-Phase Preparation of Anthracene (C14H10) via Spiroaromatic Radical Transients in the Indenyl-cyclopentadienyl Radical-Radical Reaction, JACS, 145, 3084-3091 (2023). (PDF)
75. L. B. Tuli, S. J. Goettl, A. M. Turner, A. H. Howlader, P. Hemberger, S. F. Wnuk, T. Guo, A. M. Mebel, R. I. Kaiser, Gas Phase Synthesis of the C40 Nano Bowl C40H10 Nat. Commun., 14, 1527 (2023). (PDF)
76. A. M. Mebel, M. Agúndez, J. Cernicharo, R. I. Kaiser, Elucidating the Formation of Ethynylbutatrienylidene (HCCCHCCC; X1A') in the Taurus Molecular Cloud (TMC-1) via the Gas-Phase Reaction of Tricarbon (C3) with the Propargyl Radical (C3H3) Astrophys. J. Lett., 945, L40 (2023). (PDF)
77. C. He, R. I. Kaiser, W. Lu, M. Ahmed, V. S. Krasnoukhov, P. S. Pivovarov, M. V. Zagidullin, V. N. Azyazov, A. N. Morozov, A. M. Mebel, Unconventional Gas-Phase Preparation of the Prototype Polycyclic Aromatic Hydrocarbon Naphthalene (C10H8) via the Reaction of Benzyl (C7H7) and Propargyl (C3H3) Radicals Coupled with Hydrogen-Atom Assisted Isomerization, Chem. Sci., 14, 5369-5378 (2023). (PDF)
78. S. J. Goettl, L. B. Tuli, A. M. Turner, Y. Reyes, A. H. Howlader, S. F. Wnuk, P. Hemberger, A. M. Mebel, R. I. Kaiser, Gas-Phase Synthesis of Coronene through Stepwise Directed Ring Annulation, J. Am. Chem. Soc., 145, 15443-15455 (2023). (PDF)
79. S. J. Goettl, Z. Yang, S. Kollotzek, D. Paul, R. I. Kaiser, A. Somani, A. Portela-Gonzalez, W. Sander, A. A. Nikolayev, V. N. Azyazov, A. M. Mebel, Exploring the Chemical Dynamics of Phenylethynyl Radical (C6H5CC; X2A1) Reactions with Allene (H2CCCH2; X1A1) and Methylacetylene (CH3CCH; X1A1), J. Phys. Chem. A, 127, 5723-5733 (2023). (PDF)
80. I. A. Medvedkov, A. A. Nikolayev, C. He, Z. Yang, A. M. Mebel, R. I. Kaiser, A combined experimental and computational study on the reaction dynamics of the 1-propynyl (CH3CC, X2A1) – propylene (CH3CHCH2, X2A′) system: formation of 1,3-dimethylvinylacetylene (CH3CCCHCHCH3, X1A′) under single collision conditions, Molecular Physics, e2234509 (2023). (PDF)
81. Z. Yang, G. R. Galimova, C. He, S. J. Goettl, D. Paul, W. Lu, M. Ahmed, A. M. Mebel, X. Li, R. I. Kaiser, Gas-Phase Formation of the Resonantly Stablized 1-Indenyl (C9H7•) Radical in the Interstellar Medium, Science Advances, 9, eadi5060 (2023). (PDF)
82. W. Li, J. Yang, L. Zhao, D. Couch, M. S. Marchi, N. Hansen, A. N. Morozov, A. M. Mebel, R. I. Kaiser, Gas-Phase Preparation of Azulene (C10H8) and Naphthalene (C10H8) via the Reaction of the Resonantly Stabilized Fulvenallenyl (C7H5•) and Propargyl (C3H3•) Radicals, Chem. Sci., 14, 9795-9805 (2023). (PDF)
83. Z. Yang, I. A. Medvedkov, S. J. Goettl, R. I. Kaiser, Low-Temperature Gas-Phase Formation of Methanimine (CH2NH; X1A') – the Simplest Imine - under Single-Collision Conditions, J. Phys. Chem. Lett., 14, 8500-8506 (2023). (PDF)
84. I. A. Medvedkov, A. A. Nikolayev, C. He, Z. Yang, A. M. Mebel, R. I. Kaiser, One Collision—Two Substituents: Gas-Phase Preparation of Xylenes under Single-Collision Conditions, Angew. Chem. Int. Ed., 63, e202315147 (2024). (PDF)
85. S. J. Goettl, A. M. Turner, B.-J. Sun, A. H. H. Chang, P. Hemberger, R. I. Kaiser, Gas-Phase Preparation of the Dibenzo[e,l]pyrene (C24H14) Butterfly Molecule via a Phenyl Radical-Mediated Ring Annulation, Chem. Commun., 60, 1404-1407 (2024). (PDF)
86. I. A. Medvedkov, A. A. Nikolayev, Z. Yang, S. J. Goettl, A. M. Mebel, R. I. Kaiser, Elucidating the Chemical Dynamics of the Elementary Reactions of the 1-Propynyl Radical (CH3CC; X2A1) with 2-Methylpropene ((CH3)2CCH2; X1A1), Phys. Chem. Chem. Phys., 26, 6448–6457 (2024). (PDF)
87. S. J. Goettl, C. He, Z. Yang, R. I. Kaiser, A. Somani, A. Portela-Gonzales, W. Sander, B.-J. Sun, S. Fatimah, K. P. Kadam, A. H. H. Chang, Unconventional Gas-Phase Synthesis of Biphenyl and its Atropisomeric Methyl-Substituted Derivatives, Phys. Chem. Chem. Phys., 26, 18321-18332 (2024). (PDF)
88. C. Y. Wang, L. Zhao, R. I. Kaiser, Gas-Phase Preparation of the 14π Hückel Polycyclic Aromatic Anthracene and Phenanthrene Isomers (C14H10) via the Propargyl Addition-BenzAnnulation (PABA) Mechanism, ChemPhysChem, 25, e202400151 (2024). (PDF)
89. A. M. Mebel, W. Li, L. P. Maffei, C. Cavallotti, A. N. Morozov, C.-Y. Wang, J.-Z. Yang, L. Zhao, R. I. Kaiser, Fulvenallenyl Radical (C7H5)-Mediated Gas-Phase Synthesis of Bicyclic Aromatic C10H8 Isomers: Can Fulvenallenyl Efficiently React with Closed-Shell Hydrocarbons?, J. Phys. Chem. A, 128, 5707-5720 (2024). (PDF)
90. Z. Yang, C. He, S. J. Goettl, A. M. Mebel, P. F. G. Velloso, M. O. Alves, B. R. L. Galvão, J.-C. Loison, K. M. Hickson, M. Dobrijevic, X. Li, R. I. Kaiser, Low-temperature formation of pyridine and (iso)quinoline via neutral–neutral reactions, Nat. Astron., 8, 856-864 (2024). (PDF)
91. Z. Yang, I. A. Medvedkov, S. J. Goettl, A. A. Nikolayev, A. M. Mebel, X. Li, R. I. Kaiser, Low-temperature gas-phase formation of cyclopentadiene and its role in the formation of aromatics in the interstellar medium, PNAS, 121, e2409933121 (2024). (PDF)
92. I. A. Medvedkov, Z. Yang, A. A. Nikolayev, S. J. Goettl, A. K. Eckhardt, A. M. Mebel, R. I. Kaiser, Binding the Power of Cycloaddition and Cross-Coupling in a Single Mechanism: An Unexpected Bending Journey to Radical Chemistry of Butadiynyl with Conjugated Dienes, J. Phys. Chem. Lett., 16, 658-666 (2025). (PDF)
93. Z. Yang, K. Fujioka, G. R. Galimova, I. A. Medvedkov, S. J. Goettl, R. Sun, A. M. Mebel, R. I. Kaiser, Directed Gas-Phase Formation of Azulene (C10H8): Unraveling the Bottom-Up Chemistry of Saddle-Shaped Aromatics, ACS Cent. Sci., 11, 322-330 (2025). (PDF)
94. I.A. Medvedkov, Z. Yang, S. J. Goettl, R. I. Kaiser, Identification of the elusive methyl-loss channel in the crossed molecular beam study of gas-phase reaction of dicarbon molecules (C2; X1Σg+/a3Πu) with 2-methyl-1,3-butadiene (C5H8 ; X1 A′), J. Phys. Chem. A, 129, 3280-3288 (2025). (PDF) (Supplemental Information)
95. I.A. Medvedkov, A. A. Nikolayev, Z. Yang, S. J. Goettl, A.A. Kuznetsova, A.K. Eckhardt, A.M. Mebel, R. I. Kaiser, A Combined Crossed Molecular Beam and Theoretical Investigation of the Elementary Reaction of Tricarbon (C3(X1Σg+)) with Diacetylene (C4H2(X1Σg+)): Gas Phase Formation of the Heptatriynylidyne Radical (l-C7H(X2Π)), J. Phys. Chem. A, 129, 3931-3939 (2025). (PDF) (Supplemental Information)
96. S. J. Goettl, A. M. Turner, V. S. Krasnoukhov, V. N. Azyazov, K. Kanayama, P. Hemberger, A. M. Mebel, R. I. Kaiser, Gas-Phase Synthesis of Anthracene and Phenanthrene via Radical-Radical Induced Ring Expansions, Sci. Adv., 11, eadv0692 (2025). (PDF)
97. S. J. Goettl, M. Ahmed, A. M. Mebel, R. I. Kaiser, Molecular Mass Growth Processes to Polycyclic Aromatic Hydrocarbons through Radical–Radical Reactions Exploiting Photoionization Reflectron Time-of-Flight Mass Spectrometry, Acc. Chem. Res., 58, 2682-2694 (2025). (PDF)
98. I. A. Medvedkov, A. A. Nikolayev, S. J. Goettl, Z. Yang, A. M. Mebel, R. I. Kaiser, From the laboratory to space: unveiling isomeric diversity of C5H2 in the reaction of tricarbon (C3, X1Σg+) with the vinyl radical (C2H3, X2A′), Chem. Sci., 16, 17869-17866 (2025). (PDF) (Supplemental Information)
99. S. J. Goettl, I. A. Medvedkov, A. A. Nikolayev, C. He, Z. Yang, A. M. Mebel, A. Somani, A. Portela-Gonzalez, W. Sander, R. I. Kaiser, Gas-Phase Synthesis of Naphthalene Through an Unconventional Thermal Alkyne-Alkene [2+2] Cycloaddition Mechanism, Chem. Sci., 16, 22621−22629 (2025). (PDF)
100. Z. Yang, G.R. Galimova, C. He, S. J. Goettl, A. M. Mebel, R. I. Kaiser, Exploring the Dark Radical Chemistry in Dense Molecular Cloud: Directed Gas-Phase Formation of Naphthyl Radicals, J. Am. Chem. Soc., 147, 47359–47369 (2025). (PDF) (Supplemental Information)
101. S. J. Goettl, A. G. Hartwig, Z. Yang, A. M. Mebel, R. I. Kaiser, Directed Gas-Phase Formation of the 1-Cyanovinyl Radical (H2CCCN, X2A') in the Interstellar Medium, J. Phys. Chem. A, 130, 242-249 (2026). (PDF)

